US Pharm. 2018:(43(2)27-31.
ABSTRACT: The emergence of immunotherapy is considered one of the most exciting areas in cancer treatment. With immunotherapy, durable, complete remissions are attained by inducing an immune response against various cancers. Immunotherapy is thought to have fewer side effects than traditional cancer treatments because of its mechanism of action, and is a potential option for those who have cancer that is resistant to chemotherapy and/or radiation. It is important for pharmacists to be knowledgeable about these advancements in cancer immunotherapy and be confident in their ability to apply this knowledge in clinical practice.
Cancer is a significant health problem worldwide, with its global burden expected to exceed 21 million new cancer cases and 13 million cancer deaths by the year 2030.1 The treatment of cancer depends on cancer type, severity, and several other patient-specific factors. Common cancer treatments may include surgery, chemotherapy, radiation therapy, targeted therapy, and immunotherapy.2
Immunotherapy is considered one of the most exciting areas of new discoveries and treatments for cancer.3 The recent success of several immunotherapies—for melanoma, non–small cell lung cancer, urothelial cancer, and certain solid tumors—has stimulated the development of this modality and pushed it towards the forefront of cancer treatment. Some cancer experts believe that immunotherapy treatments have the potential to fight cancer more effectively than traditional therapies, with the potential for fewer side effects.3-5
History
William Coley, known as the father of immunotherapy, discovered the concept in 1893 when he injected a mixture of selected bacteria into patients with tumors as a method of treatment. Dr. Coley developed this concept after identifying a patient who underwent unsuccessful operations to remove a large sarcoma and subsequently acquired a severe infection caused by Streptococcus bacteria. The patient’s prognosis for survival appeared grim; however, the patient survived and the cancer disappeared.3-5
After reviewing several cases with similar outcomes, Dr. Coley hypothesized that it was the bacteria that caused the eradication of the tumors; he then developed toxins, as the mixture of selected bacteria was called, to inject into certain patients. Dr. Coley experienced inconsistent success with his treatment. Clinical interest in toxins diminished in favor of radiotherapy and chemotherapy because they were associated with more predictable outcomes.4,5
Following Dr. Coley’s death, a further understanding of the immune system was established. It was only then that Dr. Coley’s findings were better grasped. Further studies determined that the toxins he utilized acted as immune stimulants. When administered, they essentially boosted the patients’ immune system, which in turn resulted in the killing of cancer cells.5
Immune System
The immune system is composed of groups of diverse cells that protect the body from foreign substances. This system recognizes “non-self” substances as foreign, while activating itself when it senses intruders.6 During the past few decades, considerable progress has been made in understanding how cancers evade the immune system. Those discoveries have yielded new methods to halt cancer immune evasion, resulting in the elimination of cancer cells.4,6,7
Immunotherapy
Immunotherapy, also referred to as biologic therapy or biotherapy, is an area of cancer treatment that uses the ability of an individual’s immune system to fight cancer.5 Cancer experts believe that these treatments can enhance the efficacy of drug treatments and reduce or eliminate the often-debilitating adverse drug effects that come with traditional chemotherapy.2 These therapies include cytokines, cancer vaccines, and most notably, certain monoclonal antibodies identified as checkpoint inhibitors, the focus of this article, because of the interest generated by their recent success.8
Checkpoint Inhibitors
By working with the intricacies of tumor pathology and immune response, checkpoint inhibitors have emerged as novel therapy options for cancer treatment. Checkpoint inhibitors are currently categorized into three groups: programmed cell death-1 (PD-1); PD-ligand 1 (PD-L1); and cytotoxic T-lymphocyte– associated antigen 4 (CTLA-4) inhibitors.
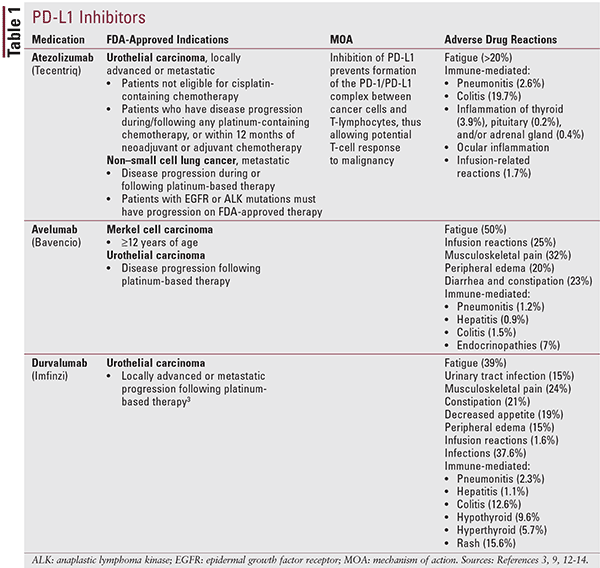
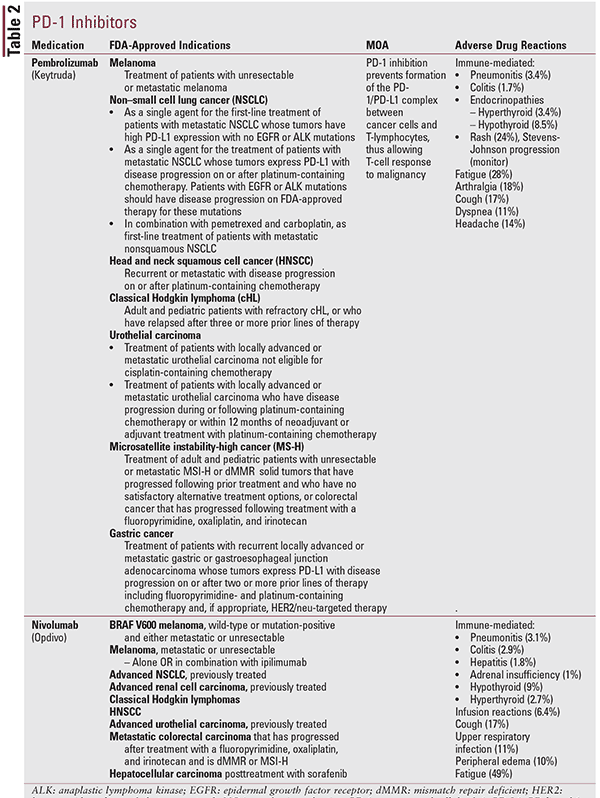
PD-L1 Inhibitors/PD-1 Inhibitors
PD-1 is a transmembrane protein expressed on the surface of circulating T-cells, B-cells, and NK-cells and is used to recognize “self” antigens from “non-self.”9,10 The binding of PD-L1, an inhibitory ligand, expressed on various cancer cells, to PD-1 receptors on immune effector cells inhibits the immune effector cells from attacking the tumor cells.9,10 PD-1 and PD-L1 inhibitors have been designed to prevent the formation of this complex and enable immune cells to attack tumor cells.9,10
Based on increasing treatment-response rates, PD-1 and PD-L1 (Tables 1 and 2) inhibitors are being approved for various types of cancer, including melanoma, non–small cell lung cancer, urothelial cancer, and most notably, solid tumors with microsatellite instability-high (MSI-H) mutations and mismatch repair deficient (dMMR) mutations. The approval of pembrolizumab in 2015 for MSI-H or dMMR solid tumors was the first time a drug has been approved based on a biomarker test for a trait and not an anatomical tumor site; this represents a significant shift in cancer treatment.11
PD-L1 inhibitors/PD-1 inhibitors are generally well tolerated; however, patients can potentially experience serious and possibly life-threatening autoimmune-related adverse effects.10 Though any organ system can be subjected to an autoimmune reaction, most commonly observed reactions are colitis, hepatitis, endocrinopathies (thyroid and adrenals), pneumonitis, and skin-rash progression to Stevens-Johnson syndrome.10,12-15
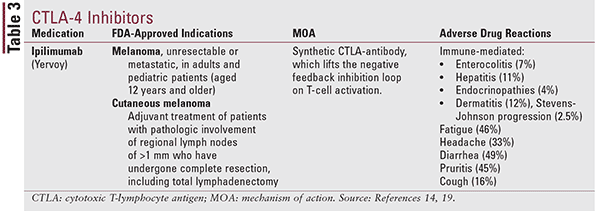
CTLA-4 Inhibitors
In addition to inhibition of the programmed cell death complex, other inhibitory modulators, such as the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) have proven to be worthwhile targets in immunotherapy. Inhibitory effects of CTLA-4 occur in the priming phase of the immune response by interfering with signals required for T-cell activation.10,16 Binding of CTLA-4 to CD80/86 inhibits the activation of T-lymphocytes, resulting in a negative feedback signal that decreases immune response.1 The drug ipilimumab induces T-cell activation by disabling the CTLA-4 feedback inhibition and allowing the immune system to activate.10,16
Ipilimumab (Table 3) is currently FDA approved for two distinct melanoma indications.17 It has been shown to demonstrate a synergistic effect with increased T-cell priming via CTLA-4 inhibition in the lymph nodes and cytotoxic activity via PD-1 inhibition in the tumor environment.10,16 Clinically significant adverse effects include immune-mediated enterocolitis, hepatitis, and endocrinopathies with adverse effects such as fatigue, diarrhea, pruritus, rash, and colitis also being common. Most immune-related events are reversible with immunosuppressive steroid treatment.10,17
Role of the Pharmacist
The growing evidence from oncology research continues to discover ways to further activate the immune system and/or bypass cancer resistance. As medication experts, it is important for pharmacists to stay informed on this topic in order to adopt strategies to counsel and assist patients and providers utilizing immunotherapy that can lead to increased adherence and improved treatment outcomes.
Pharmacists play an essential role in the management of adverse effects associated with the use of immunotherapy. Effective management relies on early recognition and prompt intervention with a correct plan of action. Several common adverse effects associated with immunotherapy (rash, diarrhea, etc.) may prompt OTC management; however, it is imperative that the patient does not rely solely on OTC medications. These adverse effects are directly related to an overactive immune system and require corticosteroid treatment for proper management. The ability of the pharmacist to counsel patients on short- and long-term use of corticosteroids and their adverse effects is also paramount.
Conclusion
The recent success of several immunotherapies, including checkpoint inhibitors, has highlighted immunotherapy as a treatment for cancer. These treatments have demonstrated efficacy in many cancers and may reduce the adverse effects that accompany traditional chemotherapy. An informed pharmacist can counsel patients and assist providers in improving adherence and treatment outcomes.
REFERENCES
1. American Cancer Society. Global cancer facts & figures. www.cancer.org/research/cancer-facts-statistics/global.html. Accessed July 16, 2017.
2. American Cancer Society. Treatment types. www.cancer.org/treatment/treatments-and-side-effects/treatment-types.html. Accessed July 16, 2017.
3. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335-3337.
4. Meiliana A, Nurrani NM, Wijaya A. Cancer immunotherapy: a review. Indones Biomed J. 2016;8(1):1-20.
5. Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73.
6. Rosenberg SA. The emergence of modern cancer immunotherapy. Ann Surg Oncol. 2005:12(5):344-346.
7. Mellstedt H, Gaudernack G, Gerritsen WR, et al. Awareness and understanding of cancer immunotherapies in Europe. Hum Vaccin Immunother. 2014;10(7):1828-1835.
8. American Cancer Society. What is immunotherapy? www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/what-is-immunotherapy.html. Accessed July 28. 2017.
9. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive-non-small cell lung cancer. N Engl J Med. 2016;375:1823-1833.
10. Plimack E, Michaelson M, Agarwal N, et al. Expert guidance for optimizing immune checkpoint inhibitor therapy for kidney and bladder cancer. National Comprehensive Cancer Center Continuing Education. Orlando, FL: March 24, 2017.
11. The Bloomberg-Kimmel Institute for Cancer Immunotherapy: The John Hopkins University, The Johns Hopkins Hospital, and Johns Hopkins Health System. Immunotherapy research timeline. www.hopkinsmedicine.org/kimmel_cancer_center/centers/bloomberg_kimmel_institute_for_cancer_immunotherapy/research/immunotherapy_research_timeline.html. Accessed: September 2, 2017.
12. Tecentriq (atezolizumab) package insert. South San Francisco, CA: Genentech, Inc.; 2016.
13. Bavencio (avelumab) package insert. Darmstadt, Germany: Merck KGaA; EMD Serono, Inc.; and Pfizer Inc.; 2017.
14. Imfinzi (durvalumab) package insert. Cambridge, England: AstraZeneca UK Limited; AstraZeneca Pharmaceuticals LP; 2017.
15. Opdivo (nivolumab) package insert. Princeton, NJ: Bristol-Myers Squibb Company; 2018.
16. Antonia SJ, Vansteenkiste JF, Moon E. Immunotherapy: beyond anti-PD-1 and anti-PD-L1 therapies. Am Soc Clin Oncol Educ Book. 2016;35:e450-e458.
17. Yervoy (ipilimumab) package insert. Princeton, NJ: Bristol-Myers Squibb Company; 2017.
18. Speiss P. Agarwal N, Bangs R, et al. (2017). NCCN Guidelines Version 5.2017 Bladder cancer, Version 5.2017 Fort Washington, PA: National comprehensive cancer network. www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed September. 2, 2017.
19. Keytruda (pembrolizumab) package insert. Kenilworth, NJ: Merck Sharp & Dohme Corp; 2016.
To comment on this article, contact rdavidson@uspharmacist.com.