US Pharm. 2020;45(11):HS1-HS8.
ABSTRACT: Incidences of hyperglycemia and ketoacidosis are higher in patients with diabetes who have coronavirus disease 2019. Frequent glucose monitoring in these patients increases the exposure risk for healthcare workers and requires the use of limited supplies of personal protective equipment. In April 2020, the FDA stated that it would not object to the use of continuous glucose monitoring (CGM) devices in the inpatient setting during the pandemic. CGM use enables real-time glucose monitoring and minimizes frequent finger-stick testing, providing an opportunity to use a new method of diabetes management in the inpatient setting. Ongoing research and FDA clearance are necessary before CGM implementation in the hospital setting can become widespread.
Continuous glucose monitor (CGM) technology has advanced quickly in recent years, transforming the management of diabetes. CGM use in the outpatient setting is increasing because of the robust data that CGM provides practitioners and the improvement in patients’ quality of life over frequent finger-stick blood glucose (BG) monitoring. Recent healthcare changes necessitated by coronavirus disease 2019 (COVID-19) have introduced an opportunity for using CGM technology to improve patient care and safety in the inpatient setting. In April 2020, the FDA stated that it would not object to CGM use in the hospital setting during the COVID-19 pandemic.1 This article will review CGM technology, discuss CGM use in the hospital setting, and address barriers to implementing CGM-based workflow.
Overview of CGM Technology
Currently available CGM devices fall into two categories: professional and personal (TABLE 1).2-6 Professional CGMs use office-provided equipment for short-term investigation into a patient’s BG control. The patient wears the sensor for up to 2 weeks, depending on the sensor used, and the data are then downloaded by the provider.2 The provider can use the short-term CGM data to gather information about the patient’s overall BG control, including rates of occurrence and patterns of hypoglycemia and hyperglycemia, time in therapeutic range, and glucose variability. Professional CGMs can be a useful tool in patients who do not qualify for personal CGM use or who are not interested in long-term use of a personal CGM.2
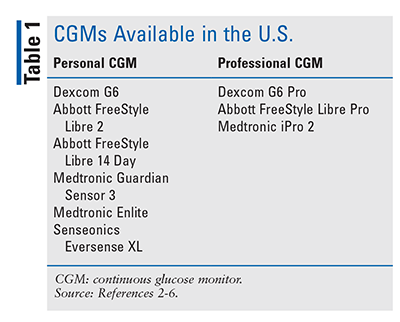
Personal CGM devices allow patients to monitor BG without routine finger sticks. Two types of personal CGMs are available: real-time CGM (rtCGM) and intermittently scanned CGM (isCGM). rtCGM devices continuously transmit glucose values to a receiver or compatible device in real time, enabling patients to make ongoing decisions about their BG treatment. isCGM devices allow patients to scan a sensor to receive BG data in order to guide treatment decisions but do not continuously transmit data unless the patient scans the sensor.2
Some important technological elements should be considered when a CGM device is used. CGMs measure interstitial glucose concentrations, whereas finger-stick testing measures capillary BG concentrations.2,7 There may be discrepancies between values obtained from a CGM device via interstitial fluid and the finger-stick BG value. These differences can be attributed to instrumental delays in measurement and physiological delays in glucose movement through the different compartments of the body.7 The differences in glucose values obtained from interstitial fluid and blood are most notable when BG concentrations are rapidly changing.2,7
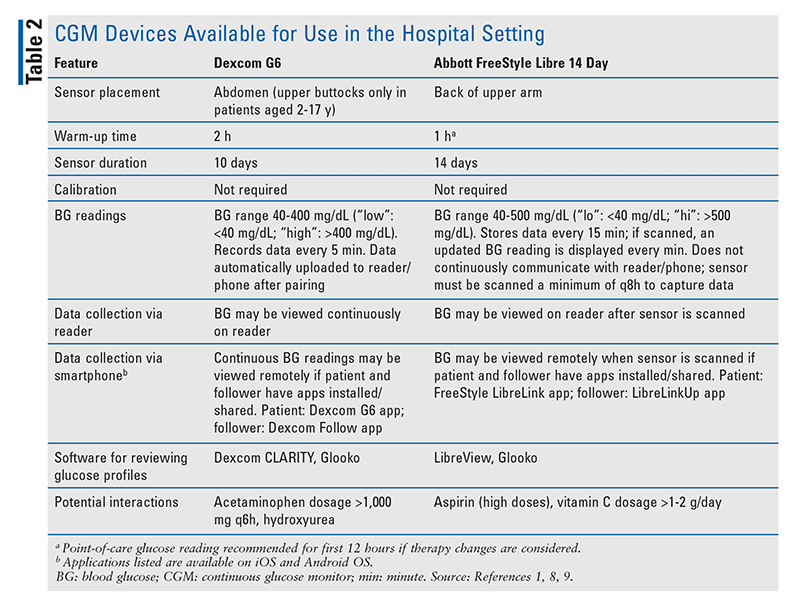
This article will focus on the two CGM systems that currently have programs to provide access to CGM technology in the hospital setting: Dexcom G6 (an rtCGM) and Abbott FreeStyle Libre 14 Day (an isCGM).3,4 These devices and their capabilities are reviewed in TABLE 2.8,9
Inpatient CGM Use
The incidence of hyperglycemia and ketoacidosis is higher in patients with diabetes who have COVID-19. Critically ill patients with hypoglycemia and glycemic variability have an increased risk of mortality.10 Improved glycemic control may reduce hospital complications and length of stay. In critically ill patients, the currently recommended target glucose range is 140 mg/dL to 180 mg/dL.11 This is difficult to achieve and typically requires nursing staff to monitor finger-stick BG readings multiple times per day and to administer subcutaneous insulin regularly. Current practice shows BG only at a certain time and does not indicate the velocity or direction in changing BG values. CGM devices can supply information on changing glucose and provide alerts for hypoglycemic or hyperglycemic events sooner than with self-monitored BG. CGM data are robust in the ambulatory setting, but in the inpatient setting they are limited by small sample sizes and studies that focus on CGM accuracy rather than clinical outcomes.
CGM accuracy in comparison with BG values is described by the mean absolute relative difference (MARD). The MARD is the average of absolute error between CGM values and matched reference values. A lower MARD indicates that the CGM readings are closer to reference glucose values, whereas a larger number indicates greater discrepancies.12 The MARD for Dexcom G6 is 9% and that for FreeStyle Libre 14 Day is 9.4% in patients with currently approved indications.8,9
The MARD may be different when CGM devices are used in hospitalized patients. A study of FreeStyle Libre 14 Day use in eight critically ill patients with type 2 diabetes showed a MARD of 14% when 274 sensor glucose readings were paired with arterial glucose concentrations or capillary glucose concentrations.13 Accuracy studies of Dexcom G6 use in critically ill patients are ongoing. In the perioperative period, studies have found that the FreeStyle Libre 14 Day system may overestimate lower glucose concentrations.14 In a study of 10 patients, Dexcom G6 showed a MARD of 9.4% in the perioperative period.15
More research will need to be conducted and FDA clearance must be obtained before CGM use in the hospital setting is widely accepted. Data collected by healthcare systems during the COVID-19 pandemic may supply additional information about the use of CGM in hospitalized patients that could expand treatment options.
Considerations for CGM Use
CGM technology offers many advantages for BG monitoring, but there are some barriers to use that are important to consider. Some key considerations for CGM use and suggestions for addressing these barriers are summarized below.
Interferences: Both the FreeStyle Libre 14 Day system and Dexcom G6 contain glucose oxidase, an enzyme that can be affected by certain medications and OTC products. With FreeStyle Libre 14 Day, high doses of aspirin may lower sensor glucose readings and high doses of ascorbic acid may falsely elevate sensor glucose readings. Low-dose aspirin does not have an interaction and should not be discontinued unless clinically necessary. Acetaminophen dosages exceeding 1,000 mg every 6 hours may interfere with sensor glucose readings for Dexcom G6 but does not impact readings of FreeStyle Libre 14 Day. In patients taking hydroxyurea, discontinuation of Dexcom G6 is recommended during therapy.8,9 Additionally, CGM accuracy may be affected by changes in skin perfusion, blood pressure, and temperature.16
Adhesive Sensitivity: Patients who are allergic to isobornyl acrylate–based adhesive should avoid the FreeStyle Libre 14 Day system and use Dexcom G6 instead.17
Site Infections: To reduce the occurrence of site infections, education should be provided regarding correct CGM placement and when to replace sensors. Proper use and placement reduce the risk of these infections.18
Procedures/Imaging: Hospitalized patients may need imaging procedures. Sensors must be removed for MRI, CT scans, or diathermy.8,9 A lead apron may be placed over the sensor during x-rays, angiograms, or fluoroscopy. Ultrasound imaging does not require the removal of CGM sensors.1
Hypoglycemia and Hyperglycemia Alerts: FreeStyle Libre 14 Day does not have alarms for hypoglycemia or hyperglycemia, whereas Dexcom G6 provides alerts for BG readings outside the preset range. Although the FreeStyle Libre 14 Day system continuously measures interstitial glucose concentrations, readings are available only when the sensor is scanned with a reader or smartphone. To minimize gaps in data collection, the FreeStyle Libre 14 Day sensor must be scanned at least once every 8 hours, so either the patient or the staff must scan the sensor with a smartphone or reader. In hospitalized patients, both Dexcom G6 and FreeStyle Libre 14 Day require the hospital to supply the patient with a reader if he or she does not have a smartphone. Patients with severe hypoglycemia or hypoglycemia unawareness may benefit from Dexcom G6’s alarms. However, frequent false alarms may limit the use of this feature. The alert settings may need to be modified for patients in order to minimize alarm fatigue.8,9,18
Education and Training: Adequate resources are necessary to provide education on these devices and to determine who is responsible for managing them. Some hospitals do not have access to endocrinology or diabetes services; in such cases, additional resources are needed to provide education. Healthcare systems might consider asking the CGM manufacturer to assist in providing staff education. Some healthcare systems have taped paper instructions outside patients’ doors to supplement staff education.18
Analyzing and Interpreting Data: The use of software or Web-based applications should be streamlined to process data collection. Education is necessary regarding the CGM trend arrows and how often and when to treat glucose readings. Too-frequent administration of bolus insulin based on the trend arrows could result in insulin stacking. Insulin stacking, which is when an insulin dose is injected while the previous insulin dose is still active, can cause hypoglycemia. The use of point-of-care testing is recommended with CGM in certain circumstances. Finger-stick BG readings are required during the warm-up period, when indicated by the CGM reader or smartphone, and any time the patient’s symptoms do not correlate with the CGM reading.8,9,18
Security and Privacy: Because CGM devices transmit information wirelessly, they may be susceptible to hacking or medical hijacking, presenting security concerns.18 Privacy is also a concern if a CGM reader is in the patient’s room or on the door of the room because it may be possible for other people to view this information. If BG readings are being transmitted wirelessly to clinical staff, guidelines should be in place to ensure that the information is documented for the correct patient.
Recommendations for Implementation
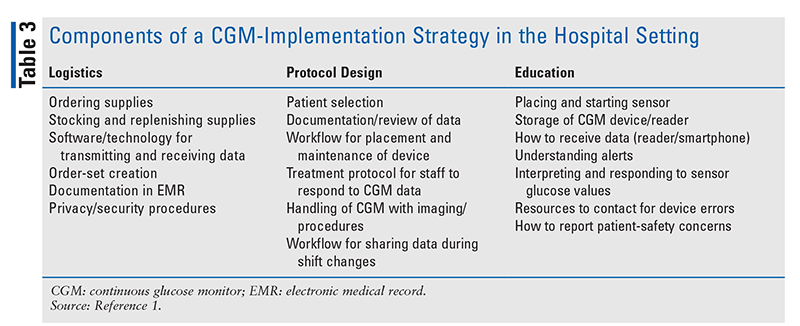
Successful implementation of CGM technology in the hospital setting requires thorough planning, education, and collaboration. Before implementing CGM technology, a hospital must form a multidisciplinary team composed of providers and hospital leadership in order to develop an effective implementation strategy. Some of the key areas to be addressed in an implementation strategy are listed in TABLE 3.1
Resources exist for hospitals that are considering CGM implementation. One of these, the collaborative open-access virtual database for COVID-19 in diabetes, allows healthcare systems to share protocols regarding management of inpatient diabetes and diabetes technology, including the implementation of CGMs within a system.19 Hospitals interested in introducing CGM use should contact the manufacturer regarding available programs for supplying sensors to hospitals.3,4
Conclusion
COVID-19 has forced health systems to develop strategies for adapting to the unique needs of patients and staff during the pandemic. Frequent monitoring of glucose concentration in patients with diabetes increases the exposure risk for healthcare workers and requires the use of limited supplies of personal protective equipment. Implementing CGM technology in the hospital setting could be one strategy to improve patient care and ensure the safety of staff and patients. An understanding of CGM technology and its limitations is vital in the decision to utilize CGM technology. A successful implementation strategy should be developed by a multidisciplinary team with a focus on logistics, protocol design, and education. The emergent need for CGM technology during the COVID-19 pandemic has created an opportunity to evaluate and research this technology in the hospital setting. Ongoing research and FDA clearance are necessary before CGM implementation in the hospital setting can become widespread.
REFERENCES
1. Galindo RJ, Aleppo G, Klonoff DC, et al. Implementation of continuous glucose monitoring in the hospital: emergent considerations for remote glucose monitoring during the COVID-19 pandemic. J Diabetes Sci Technol. 2020;14(4):822-832.
2. Longo R, Sperling S. Personal versus professional continuous glucose monitoring: when to use which on whom. Diabetes Spectr. 2019;32(3):183-193.
3. Dexcom continuous glucose monitoring. www.dexcom.com/#. Accessed August 10, 2020.
4. The FreeStyle Libre 14-day system. www.freestylelibre.us/system-overview/freestyle-14-day.html. Accessed August 10, 2020.
5. Eversense CGM. www.eversensediabetes.com. Accessed August 10, 2020.
6. Continuous glucose monitoring. www.medtronicdiabetes.com/treatments/continuous-glucose-monitoring. Accessed August 10, 2020.
7. Petrie JR, Peters AL, Bergenstal RM, et al. Improving the clinical value and utility of CGM systems: issues and recommendations: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care. 2017;40(12):1614-1621.
8. Dexcom product guides. www.dexcom.com/guides. Accessed August 10, 2020.
9. FreeStyle Libre 14 day system user guide. www.freestylelibre.us/support/overview.html. Accessed August 10, 2020.
10. Krinsley JS, Meyfroidt G, van den Berghe G, et al. The impact of premorbid diabetic status on the relationship between the three domains of glycemic control and mortality in critically ill patients. Curr Opin Clin Nutr Metab Care. 2012;15(2):151-160.
11. American Diabetes Association. 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43(suppl 1):S193-S202.
12. Heinemann L, Schoemaker M, Schmelzeisen-Redecker G, et al. Benefits and limitations of MARD as a performance parameter for continuous glucose monitoring in the interstitial space. J Diabetes Sci Technol. 2020;14(1):135-150.
13. Ancona P, Eastwood GM, Lucchetta L, et al. The performance of flash glucose monitoring in critically ill patients with diabetes. Crit Care Resusc. 2017;19(2):167-174.
14. Schierenbeck F, Franco-Cereceda A, Liska J. Accuracy of 2 different continuous glucose monitoring systems in patients undergoing cardiac surgery. J Diabetes Sci Technol. 2017;11(1):108-116.
15. Nair BG, Dellinger EP, Flum DR, et al. A pilot study of the feasibility and accuracy of inpatient continuous glucose monitoring. Diabetes Care. 2020;43(11):e168-169.
16. Dungan KM, Han W, Miele A, et al. Determinants of the accuracy of continuous glucose monitoring in non-critically ill patients with heart failure or severe hyperglycemia. J Diabetes Sci Technol. 2012;6(4):884-891.
17. Oppel E, Kamann S, Reichl FX, Högg C. The Dexcom glucose monitoring system—an isobornyl acrylate-free alternative for diabetic patients. Contact Dermatitis. 2019;81(1):32-36.
18. Thompson B, Leighton M, Korytkowski M, Cook CB. An overview of safety issues on use of insulin pumps and continuous glucose monitoring systems in the hospital. Curr Diab Rep. 2018;18(10):81.
19. Collaborative open-access virtual database for COVID-19 in diabetes. www.covidindiabetes.org. Accessed August 14, 2020.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





