US
Pharm. 2006;2:28-33.
As community retail pharmacy
begins working with Medicare beneficiaries to implement the Part D
prescription drug benefit launched earlier this
year, new challenges for pharmacy are on the horizon. The Federal Deficit
Reduction Act of 2005 will change the way that Medicaid pays for generic
prescription medications on January 1, 2007.1 Other provisions of
the act take effect in the middle of 2006 and will result in greater pricing
transparency in the pharmaceutical market.
The legislation, which
narrowly passed the House of Representatives on December 19, 2005, and the
Senate on December 21, 2005, will reduce federal spending of many government
programs, including farm subsidies, student loans, and health care programs
such as Medicare and Medicaid, by about $40 billion over the next five years.
2 Most importantly for retail pharmacy, the legislation makes
significant reductions in Medicaid generic prescription drug payments, gives
states flexibility to impose higher premiums and cost-sharing amounts for
health care services, and allows states to offer slimmed-down health care
benefit packages to some Medicaid recipients.
Although hotly contested, the
bill's reductions in generic drug payments resulted from many key
policymakers' belief that Medicaid is overpaying pharmacies for
generic medications. During the past 18 months, several studies and reports
from the Congressional Budget Office (CBO), the Department of Health and Human
Services (HHS) Office of the Inspector General (OIG), and the Congressional
Governmental Accountability Office have led many key members of Congress to
conclude that Medicaid's average wholesale price (AWP)-based system for
reimbursing pharmacies for prescription drugs is flawed. In particular, the
studies showed that the system used by the Centers for Medicare and Medicaid
Services (CMS) to set federal upper payment limits (FULs) for generic
prescription drugs often overstates a pharmacy's true acquisition costs for
generics and does not impose FULs in a timely manner when multiple-source
drugs are on the market. As a result, these studies concluded that Medicaid
had overpaid hundreds of millions of dollars to pharmacies for generic
medications.
Retail pharmacy has disagreed
with this assessment, arguing that the studies have exaggerated pharmacy
margins on generic prescriptions by calculating "percentage" margins rather
than "actual dollar" margins. This made it appear that pharmacies were making
significantly high margins on generics given the low-cost basis of these
drugs. The retail pharmacy industry has also pointed out that Medicaid
programs reap significant financial benefits when a pharmacy dispenses a
generic medication that costs about $20 on average, versus a single-source
brand name medication that costs about $100 on average. Thus, current
incentives to dispense generic medications would be sharply reduced if
payments to pharmacies significantly decreased. At the end of the day,
however, federal policymakers decided to take aim at generic drugs, reducing
payments for these medications by $6.3 billion in combined federal and state
Medicaid spending over the next five years.
Medicaid Payment Limits for
Generic Multiple-Source Drugs
Medicaid is an
important payer for most pharmacies, representing about 7% to 8% of the
average number of prescriptions filled by a typical pharmacy. Increased use of
generic drugs has been an important part of most states' Medicaid cost
management strategies. Generic medications represent about half of all
Medicaid prescriptions dispensed, and expenditures for these drugs accounted
for about 15% of the approximately $40 billion spent on prescription drugs by
Medicaid in 2005.
The new legislation will
change how CMS determines a generic drug's FUL, the maximum amount that the
agency will reimburse states for a specific dosage form and strength of a
generic drug dispensed by pharmacists--regardless of the cost of the product to
the pharmacy. Traditionally, FULs have been set for products that have three
or more sources of supply. CMS usually sets these FULs when, in addition to
the off-patent innovator, there are two additional A-rated generic versions of
the drug on the market.
Currently, the FUL is set at
150% of the lowest published price (i.e., wholesale acquisition cost [WAC],
AWP, or direct price) for a dosage form and strength of a generic drug
product. States cannot exceed the aggregate spending on drugs that have FULs
(i.e., they can exceed the FUL on one drug as long as they pay less on another
drug with an FUL). CMS generally reviews the pharmaceutical pricing compendia
(e.g., Red Book or Blue Book) to identify the lowest published price for a
generic drug. About 600 generic products currently have an FUL. Many states
further reduce the amounts that they pay for generics by placing "maximum
allowable costs" (MACs) on generic drugs, some of which already have FULs.
States use various methods to determine their MACs. Some states create MACs
for products that have only two sources of supply rather than three. All of
these policy approaches are permissible.
The new legislation would
calculate the FUL for generic drugs based on 250% of the lowest average
manufacturer's price (AMP) for the generic in the class, rather than 150% of
the lowest published price. CMS will identify the lowest AMP of a product in a
therapeutically equivalent and bioequivalent group of generics and multiply
that number by 2.5. This amount would be the FUL for all therapeutically
equivalent and bioequivalent generic drugs within that class, including the
innovator, unless the physician writes a restrictive prescription and wants
the Medicaid recipient to have the innovator drug. However, as is the case
now, states can further reduce these new generic payment limits through their
own MACs for generic drugs.
That raises the next question:
What is AMP? The term was defined in federal law under the Omnibus
Budget Reconciliation Act of 1990 as, the average price paid to
manufacturers by wholesalers for drugs distributed to the retail class of trade
. Manufacturers calculate AMP at the end of each calendar quarter to help them
determine the amount of rebates owed to state Medicaid programs for the
manufacturer's drug products dispensed in that quarter.
In theory, AMP should
represent the manufacturer's net revenue earned per unit (e.g., tablet,
capsule) for the sale of a drug to the manufacturer's retail pharmacy
customers only. When calculating AMP, manufacturers consider all of the
rebates, discounts, and price concessions that they provide to retail pharmacy
customers, such as prompt pay discounts, volume discounts, and cash discounts.
Manufacturers have to include the value of any free goods that they provide to
retail pharmacies when calculating the AMP.
Thus, AMP doesn't truly
represent the price that pharmacies pay for a drug; it represents the net
revenue earned by the manufacturer on the sales of that drug to the retail
class of trade. In addition, the AMP doesn't account for additional costs paid
by pharmacies for wholesaler charges or other distribution system charges to
stock and inventory the product in the pharmacy. As a result, AMP would have
to be increased by a certain percentage to reflect a pharmacy's true
acquisition costs. A recent OIG report found that on average, AMP was about 6
and 59 percentage points lower than WAC for brand-name and generic drugs,
respectively.3
To illustrate how AMP is
calculated and how it would be applied in determining the FUL, suppose a
manufacturer earned $100 in revenue from the sales of 1,000 units of a drug to
all of its retail pharmacy customers but paid $20 in discounts to these
customers. The AMP for this product would be 8 cents/unit ($100 minus
$20/1,000 units). If there are three manufacturers of a dosage form and
strength of a generic drug and the AMPs for these drugs are 8 cents, 10 cents,
and 12 cents, the new FUL would be 20 cents (2.5 X 8 cents/unit = 20 cents).
How will these generic payment
reductions affect retail pharmacy economics? Since AMP data are not public,
making estimates is difficult. However, some existing studies and reports shed
light on the economic impact of these reductions. This past summer, the OIG
estimated that if Medicaid based FUL amounts on 150% of the lowest reported
AMP, rather than 150% of the lowest published price, the program may have
saved up to $300 million in just one quarter of 2004. This figure represents
75% of the $396 million spent on generic versions of FUL drugs during that
period.
When these new generic drug
payment reductions go into effect in 2007, the average Medicaid generic
prescription reimbursement will decrease by approximately $4.25, which will
reduce the average generic payments to pharmacies by about 17%. The new
pricing system could therefore dramatically reduce retail pharmacy's
incentives to dispense generics, especially if the gross margin on generic
prescriptions is less than that of brand-name prescriptions.
Further, since the new
legislation changes the definition of a "multiple-source drug," an additional
number of generics will be subject to an FUL. CMS may begin setting an FUL
when only two therapeutically equivalent and bioequivalent generic drugs are
on the market. Currently, at least three such products have to be marketed
before a drug is considered a multiple-source product for the purposes of
determining an FUL. As a result, an FUL could be set on a product immediately
after the innovator's patent expiration when only the innovator and the first
generic are on the market. This is an important change. Many generics have a
six-month period of exclusivity during which no other generic can be marketed.
Even when three generic products became available, CMS did not always
immediately set an FUL on these products.
The use of an FUL for these
generics may have a significant impact on generic profitability and on overall
pharmacy profitability. A CBO study in 2004 found that pharmacies were earning
their highest dollar margins on "newer generics."4
These generics represented 8.4% of all prescriptions in 2002 but accounted for
37% of the increase in pharmacy margins between 1997 and 2002. This is because
during the time before an FUL is set, the generic drug is generally paid for
at the same AWP discount rate that the state uses to pay for a single-source
drug (e.g., AWP minus 14%). Under the new legislation, these generic drugs
will be subject to an FUL of 250% of the product with the lower AMP. In fact,
a significant amount of the savings may come from reductions in Medicaid
payments for generics with only two sources of supply.
Transparency in Pharmaceutical
Pricing
Many federal and
state policymakers have long maligned "AWP" as "Ain't What's Paid" and have
called for more accurate reimbursements through a greater transparency in the
actual prices paid by pharmacies for prescription medications. Under this
legislation, the Secretary of HHS is required to make the most recently
reported AMP data for patented single-source and multiple-source drugs
available to states on a monthly basis, beginning in July 2006. CMS has
previously been prohibited by law from making these data public. However, such
disclosure will create greater transparency in the pharmaceutical marketplace
and provide states with additional data to determine the reimbursement rates
to pharmacies.
Under the new legislation, the
Secretary of HHS is also authorized to establish a quarterly-updated Web site
that would provide the public with AMP data on medications. This will result
in increased price transparency as pharmacy benefits managers (PBMs) and plans
are able to use AMP to compare prices and set reimbursement rates. Customers
can also use public AMPs to compare the prices they pay for medications.
While the release of AMP data
may increase transparency, it is not clear that these AMP data will be
reliable or that they will reflect the prices paid by community retail
pharmacies. It has been well documented that there is no consistency in how
manufacturers calculate AMP. For example, some manufacturers include rebates
paid to PBMs and sales to mail-order and nursing home pharmacies when
calculating AMP. Such inconsistencies have resulted because CMS has never
given manufacturers final instructions on how to calculate AMP.
To address this situation, the
legislation requires that the OIG review the requirements for the AMP and the
manner in which it is determined based on the changes made under this
legislation. This OIG review must occur no later than June 1, 2006. The OIG is
instructed to make recommendations to the Secretary of HHS and Congress on any
appropriate changes to the way that AMP is determined. The Secretary must
publicize a regulation no later than July 1, 2007, that clarifies the
requirements for the AMP and the manner in which it is determined and that
takes into consideration the OIG recommendations. However, by this time, the
AMP data will have already been available to states and to the public for a
year, and there is no guarantee that the OIG or the HHS will issue a final
rule in 2007 requiring manufacturers to calculate the AMP using only the sales
to retail pharmacies.
When states and the public
obtain these new AMP data in mid-2007, it will most likely create more pricing
pressure on retail pharmacies. States will compare their current net payment
rates for single-source drugs with the reported AMP amounts for these drugs
and determine whether they are overpaying. Consumers will compare the prices
they are paying for medications at the pharmacies with the reported AMPs and
may also conclude that they are overpaying.
New Authority for States to
Impose Premiums and Cost-Sharing
With some
exceptions, states have generally been prohibited from charging premiums and
enrollment fees to Medicaid recipients.5 Unless they have obtained
a waiver, states have been able to impose only nominal cost-sharing fees on
Medicaid recipients--between 50 cents and $3, depending on the cost of the
service provided. These cost-sharing amounts have not been updated in a very
long time. Many state policymakers have complained that these low copay
amounts, combined with the ability of Medicaid recipients to obtain services
even if they are unable to pay, renders cost-sharing an ineffective means of
encouraging Medicaid recipients to use prescription medications that are more
cost-effective.
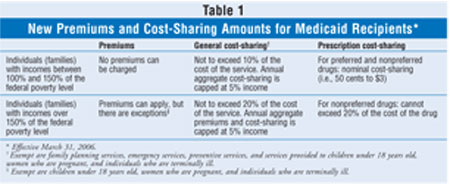
Beginning March 31, 2006, the
new legislation allows states to impose premiums on some Medicaid recipients
and to impose additional cost-sharing amounts on certain groups of Medicaid
recipients for Medicaid-covered services. States will be prohibited from
imposing premiums or enrollment fees on very poor Medicaid recipients (i.e.,
those with family incomes between 100% to 150% of the federal poverty level
[FPL]). However, states could impose a cost-sharing amount that does not
exceed 10% of the cost of the item or service. An aggregate maximum of 5% of
family income (either monthly or quarterly income, as specified by the state)
for all cost-sharing would apply.
For individuals with a family
income that exceeds 150% of the FPL, states could impose premiums and
terminate services after 60 days of nonpayment of premiums. In this income
group, states could impose cost-sharing amounts that do not exceed 20% of the
cost of the item or service. An aggregate maximum of 5% of family income
(either monthly or quarterly income, as specified by the state) for all
premium and cost-sharing amounts would apply. Populations exempt from these
new premiums include individuals younger than 18 years old, pregnant women,
and terminally ill patients in hospice care. Cost-sharing cannot be imposed on
these persons or on family planning, emergency, and preventive services.
Under the new legislation,
states may authorize providers to require payment of the cost-sharing amount
as a condition of providing the service. Providers may reduce or waive the
cost-sharing amount on a case-by-case basis. Under the current law, providers
cannot deny services to Medicaid recipients who cannot or will not pay
cost-sharing amounts. To keep pace with inflation, the Secretary of HHS would
be required, beginning in 2006, to index nominal cost-sharing amounts by the
annual percentage increase in the medical care component of the consumer price
index for all urban consumers (U.S. city average).
The legislation also creates
new cost-sharing policies for prescription drugs provided to Medicaid
recipients. Starting on or after March 31, 2006, states can designate certain
prescription drugs as "preferred" drugs, defined as drugs that are less costly
or the least costly drugs within a class. Other drugs would be designated as
"nonpreferred drugs." States can waive or charge Medicaid recipients lower
cost-sharing amounts for preferred drugs and higher cost-sharing amounts for
nonpreferred drugs.
The difference in the amount
of cost-sharing that can be charged for a nonpreferred drug compared to a
preferred drug depends on an individual's income level. For individuals with
an income below 150% of the FPL, the highest cost-sharing amount that can be
charged for nonpreferred drugs is equal to the nominal cost-sharing amounts in
existence at the time. For those individuals with an income above 150% of the
FPL, the maximum cost-sharing amount for a nonpreferred drug would be equal to
20% of the cost of the drug.
Individuals who are typically
exempt from Medicaid cost-sharing, such as children, may pay cost-sharing for
nonpreferred drugs. This amount cannot exceed the nominal cost-sharing
amounts. Cost-sharing amounts for preferred and nonpreferred drugs paid for by
an individual count toward meeting the aggregate overall 5% cost-sharing cap.
States must allow Medicaid recipients to use the nonpreferred drug at the
cost-sharing amount for the preferred drug if a physician determines that the
nonpreferred drug for the treatment of the same condition would not be
effective or would have adverse effects. These changes are summarized in table
1.
Conclusion
New changes to the
Medicaid program will have significant economic implications for community
retail pharmacy. The number of prescriptions paid for by Medicaid will
decrease with the shift of the dual-eligible beneficiaries from Medicaid to
Medicare Part D. Measures to encourage transparency for pharmaceutical pricing
have the potential to influence other larger government programs such as
Medicare Part D and third-party commercial payers.
The public release of AMP data
will allow consumers and third-party payers to compare the amounts they pay
for prescriptions with these data and create downward pressure on pricing.
Such a move will affect manufacturers, wholesalers, and retail pharmacies and
may reduce already thin margins in the retail pharmacy supply chain.
Pharmacies will compare the prices that they pay for medications to the posted
AMP and likely seek lower prices from manufacturers to reduce their
acquisition costs below the average. Will such increased transparency result
in greater uniformity in manufacturer pricing and a reduction or elimination
in multitiered manufacturer pricing? Only time will tell. One thing is
certain, though. Change is coming, and the retail pharmacy industry must be
prepared.
REFERENCES
1. House Report
109-362, H.R. 4241, S. 1932.
2. The House of
Representatives voted again to pass the bill 216-214 on February 1, 2006, as a
result of minor technical changes made by the Senate.
3. HHS-OIG. "Medicaid
Drug Price Comparisons: Average Manufacturer Price to Published Prices"
OEI-05-05-00240. June 2005.
4. Medicaid's
Reimbursements to Pharmacies for Prescription Drugs. Congressional Budget
Office. December 2004. Available at: www.cbo.gov/
ftpdocs/60xx/doc6038/12-16-Medicaid.pdf. Accessed January 12, 2006.
5. Under certain
circumstances, pregnant women and infants, and families with incomes greater
than 150% of the federal poverty level; individuals who are medically needy;
and workers with disabilities can be charged premiums, which have ranged from
$1 to $19.
To comment on this article,
contact
editor@uspharmacist.com.






