US Pharm. 2009(34). Published October 7, 2009.
Seasonal influenza occurs when influenza viruses are circulating in the community. The usual influenza season in the Northern Hemisphere is from late fall through the early spring months. In the Southern Hemisphere, seasonal influenza occurs during the summer months, and in the Tropics, influenza viruses circulate all year long.1,2 Seasonal influenza leads to 250,000 to 500,000 deaths each year worldwide.1-3Pandemic influenza is defined by increased numbers of influenza cases occurring over a longer period of time with higher mortality rates as a result of new antigenic viral composition against which the general population usually has no or low immunity. Three occurrences of pandemic influenza in the 20th century include the 1918-1919 Spanish influenza A/H1N1 pandemic, the 1957-1963 Asian influenza A/H2N2 pandemic, and the 1968-1970 Hong Kong influenza A/H3N2 outbreaks. All three influenza pandemics of the 20th century were started by bird-originated influenza A viruses.1-4 The Spanish flu pandemic alone killed over 40 million people worldwide in 1918-1919.1,5-8
Seasonal Influenza
Seasonal (interpandemic) influenza is usually a self-limiting viral infection and, in most individuals, causes fever, headache, muscle aches, and respiratory symptoms, including cough, nasal congestion, runny nose, and sore throat. Seasonal influenza can lead to hospitalization and severe morbidity and mortality in certain individuals, including the elderly, the very young, patients who are immunocompromized, and persons with certain medical conditions, including pulmonary disease, heart disease, and diabetes.1,2 Influenza infection can exacerbate these underlying conditions and can be followed by a secondary bacterial infection causing further complications, morbidity, and mortality.1,2,5-7
Flu Virus Types
There are three types of influenza viruses: A, B, and C. Influenza A virus is found in animals and humans and is often the culprit of major pandemics, while influenza B virus is found only in humans. Influenza C virus, which is rare, usually causes milder symptoms than influenza A or B and is therefore not medically treated.1,8 Influenza A viral RNA contains eight segments and is embedded in a lipid envelope surface that contains glycoprotein spikes for attaching the virus to the host cell.9 This causes viral particles to release into the host cell, leading to the host's immune response (if immunity to this particular viral antigen exists). These viral glycoprotein spikes are called hemagglutinin and neuraminidase. There are 16 different hemagglutinin (H) types and 9 neuraminidase (N) types. Influenza A virus particles also contain an M2 protein that is present in the virus membrane in limited amounts, but becomes present in high amounts in the virus-infected cells after the infection starts. The M2 protein's "tail" of influenza A viruses is relatively preserved in various viral subtypes, while mutations often occur in hemagglutinin, neuraminidase, or sometimes both.6-11 Vaccines that target and inactivate the hemagglutinin part of the virus protect people from viral infection itself. Inactivating the neuraminidase part prevents further morbidity and mortality, and targeting the M2 protein part of the virus offers the potential for universal influenza vaccine production if the M2 protein's amino acid sequence remains unchanged.10,11
Mutations and Pandemic Outbreaks
Influenza viruses are highly variable, and frequent mutations in viral genetic material (antigenic drift and antigenic shift) allow flu virus to "escape" from human immune defenses, leading to pandemic influenza outbreaks. In antigenic drift, genetic mutation of hemagglutinin occurs. Both influenza A and influenza B viruses undergo antigenic drift variations, leading to the need for updates to annual flu vaccine compositions to keep up with these changes. In antigenic shift variations, which only occur in influenza A, viruses of human origin and animal origin mutate often in an animal reservoir, forming a hybrid virus containing both mutated hemagglutinin and neuraminidase.7-10 Novel influenza A viruses of threat today include influenza A/H5N1 (bird flu) and, more recently, influenza A/H1N1 (swine flu).5-8,12-17
The H1N1 Pandemic
Based on the World Health Organization (WHO) Preparedness and Response Alert System to Global Pandemic Outbreak (TABLE 1), the H5N1 influenza (bird flu) was classified as phase 3 on the pandemic alert scale, while H1N1 influenza (swine flu) has been classified as phase 6--indicating that a global pandemic was underway. As of the September 13, 2009, WHO update, there had been 296,471 cases of laboratory-confirmed swine flu with at least 3,486 deaths reported worldwide.7,8 H1N1 is the first global pandemic outbreak of the 21st century.2,7,8,14-16 Historically, pandemic influenza outbreaks have been defined by several common characteristics, including shift in viral subtypes, higher death rates in younger individuals, successive pandemic waves, higher transmission rates when compared to seasonal influenza, and different geographic regions being impacted differently.6-8,10 National preparedness and international collaboration are the key to dealing with pandemic influenza outbreaks. Interpandemic data collections are equally important.2,7,8 The CDC's Influenza Division collects and analyzes influenza activity data year-round. A specific influenza activity report is published each year on a weekly basis from October through mid May during the peak months of influenza activity. CDC surveillance includes viruses that are circulating, including novel influenza A viruses, outpatient illnesses, mortality, hospitalizations, and viral geographic spread. CDC influenza data collection is a collaborative work of state and local health authorities, vital statistics offices, laboratories, epidemiologists, and health care providers. Based on influenza surveillance collected by the CDC, a national picture of influenza activity is available each year.2,4,5

The WHO's Role in Fighting Influenza
The WHO plays a vital role in global seasonal and pandemic influenza prevention and treatment. The WHO meets with directors of collaborating centers and reference laboratories in a global meeting twice a year (once in February to evaluate current viral strains in the Northern Hemisphere and once in September to evaluate viral strains in the Southern Hemisphere) and makes recommendations for the composition of seasonal influenza vaccine for the upcoming season. Since these meetings occur well in advance of the upcoming influenza season, mismatch between the vaccine composition and the viral strains that predominate during that particular season is possible, and could explain what happened during last year's flu season.3,8,11,14 Seasonal influenza vaccine that is administered intramuscularly contains inactivated influenza viruses, is trivalent (contains two influenza A virus strains and one influenza B virus strain), and provides host immunity against closely related strains. Live-attenuated vaccine is also available in nasal spray form, but can only be used in certain populations.3-5
Seasonal Influenza Vaccine Guidelines
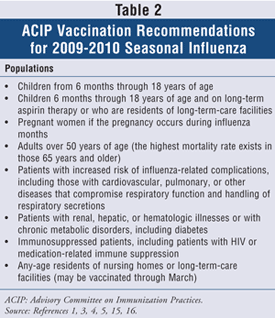
Currently, the influenza vaccine-making process can take more than 6 months because viral strains are injected into chicken eggs and allowed to grow.1,3,5,15,17-20 There are studies available that discuss the possibility of growing viral strains by means other than in chicken eggs, including vectors, viral proteins, Vero cells, and reverse genetics, but these methods of vaccine manufacturing are still under investigation.17,19-28 Annual vaccination is the most effective means of preventing influenza illness, inducing antibody response that neutralizes the virus and prevents viral spread and further infection. All children aged 6 months to 8 years who have not been vaccinated previously at any time with at least one dose of trivalent-inactivated vaccine should receive two doses of age-appropriate vaccine in the same season, with a single dose during subsequent seasons. A second vaccination is needed to provide optimal antibody response to influenza. Healthy adults 65 years of age or younger require one dose of vaccine once per year. Influenza vaccination in adults over 65 years of age is known to be less effective following single-dose vaccination than in young, healthy adults. According to some studies, increasing the initial influenza vaccine dose in elderly people who are residents of long-term-care facilities could be beneficial, but current Advisory Committee on Immunization Practices (ACIP) and Infectious Diseases Society of America (IDSA) guidelines do not recommend increased doses for preventing influenza outbreaks in the institutional setting, as collective studies did not show a better immune response after additional vaccine doses in this population.1,3-5,24
The possibility of using prepandemic influenza vaccine containing a mixture of different previous pandemic viruses could be considered to induce a cross-reactive immune response that would potentially neutralize future pandemic influenza virus. Prepandemic vaccination would lead to a prompt immune response by use of a vaccine adjuvant booster during early pandemic stages or during vaccine outages.10,19,21,23-28 Antiviral medications also play an important role in the prevention and treatment of influenza outbreaks.1,3-5,7,11,15,16
Vaccine development involves an understanding of antigen variability. Vaccine composition is updated yearly to keep up with antigenic drift; however, antigenic shift is much harder to predict, and current vaccine development does not protect against this process.5,10,11,17,25-27 The seasonal influenza vaccine-making process for the upcoming influenza season is complete, and the seasonal influenza vaccine has received FDA approval.
The seasonal influenza vaccine for the 2009-2010 season is a trivalent-inactivated vaccine containing A/Brisbane/59/2007 H1N1-like, A/Brisbane 10/2007 H3N2-like/B/Brisbane/60/2008-
Live-attenuated seasonal influenza vaccination in a nasal spray form containing the same above viral composition is available as an option for healthy children and adults aged 2 to 49 years and for nonpregnant women in the same age-group. For annual vaccination recommendations with trivalent-inactivated influenza vaccine (if no contraindications exist), refer to TABLE 2.
Vaccinating Against H1N1
The FDA has recently approved four vaccines against H1N1 2009 pandemic virus. Three of the H1N1 vaccines are available in injectable form and one is available in nasal spray form, and all four vaccines are monovalent. As with seasonal influenza vaccinations, H1N1 vaccine is contraindicated for use in patients with an allergy to eggs or to any other components of this influenza vaccine. At press time, the H1N1 vaccine was expected to be available within 4 weeks of FDA approval, or in mid October 2009.4,5,15,16,27,28
Current H1N1 vaccination recommendations call for children 9 years of age and younger to be administered two doses of the monovalent H1N1 2009 virus vaccine, as they carry no immunity to this virus. Children 10 years of age and older and adults should be administered one dose of H1N1 vaccine. Further clinical studies are under way that will provide additional information regarding this vaccination and its dosing recommendations.
The ACIP provided recommendations regarding H1N1 vaccine once it becomes available.3,4,5,14,27,28 H1N1 vaccination should occur first in the target populations as listed in TABLE 3. The ACIP acknowledged that these populations are at increased risk of complications from influenza illness, and these persons are also likely to come in contact with H1N1 influenza virus in their work environments and can possibly transmit the virus to others in health care settings. These populations are also in close contact with infants under 6 months of age or those who are too young to be vaccinated.
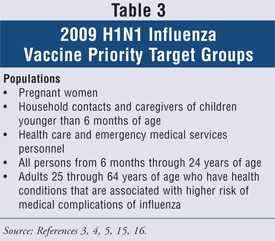
High-risk groups for complications from H1N1 influenza remain the same as high-risk groups for seasonal influenza. Vaccination against 2009 H1N1 should occur in addition to seasonal influenza vaccination. Interim recommendations on antiviral use for treatment and prophylaxis of H1N1 influenza can be found in TABLE 4. Patients with suspected novel H1N1 should only be treated with antiviral medications if they are hospitalized or at high risk of influenza-related complications, to delay emergence of virus resistance and to preserve the limited antiviral supplies. Current H1N1 virus is still susceptible to oseltamivir. If the decision is made to treat with antiviral medication, this should start within 48 hours of symptom onset. In areas of oseltamivir resistance, zanamivir or oseltamivir/rimantadine or oseltamivir/amantadine can be used. Pre-exposure prophylaxis should be considered in special circumstances and should start 7 days before contact with an H1N1 infected person and continue for 10 days after the last exposure, and the antiviral choices remain the same. If more than 7 days have passed after exposure to H1N1, then no prophylaxis is recommended at this time. Of note, oseltamivir has recently been approved for use in children less than 1 year of age, and the dosing is age-based. This indication is only for use under the Emergency Use Authorization Act. For use in pregnant women, even though no data are available at this time, oseltamivir is recommended by the ACIP as a preferred agent. Seasonal recommendations will remain in place for the 2009-2010 influenza season, but 2009 H1N1 recommendations can change depending on the viral resistance patterns and antiviral and vaccine availability. One should always consult ACIP recommendations on the CDC Web site for the most up-to-date guidelines.

The author would like to thank Corstiaan Brass, MD, Infectious Diseases, for editing pearls, and Farida Naheed, PharmD, for advice.
REFERENCES
1. Harper SA, Bradley JS, Englund JA, et al. IDSA guidelines for seasonal influenza in adults and children. Clin Infect Dis. 2009;48:1003-1042.
2. Influenza Division of CDC. Overview of influenza surveillance in the United States. A weekly influenza surveillance report. 2008-2009 influenza season. www.cdc.gov/flu. Accessed August 3, 2009.
3. World Health Organization. Recommended composition of influenza virus vaccines for use in the 2009-2010 Northern Hemisphere influenza season. www.who.int/csr/disease/
4. Recommended composition of influenza virus vaccines for use in the 2009-2010 influenza season (Northern Hemisphere winter). Wkly Epidemiol Rec. 2009; 84(9):65-72.
5. Prevention and control of seasonal influenza with vaccines. Recommendations of Advisory Committee on Immunization Practices (ACIP). Morb Mortal Wkly Rep. 2009;58(RR-08):1-56.
6. Prevention and Control of Influenza. Recommendations of Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report. 2008;57(RR-07):1-60.
7. World Health Organization. Epidemic and pandemic alert and response (EPR). Current WHO Phase of Pandemic Alert. www.who.int/csr/disease/avian influenza/phase/en. Accessed September 22, 2009.
8. World Health Organization. Global influenza surveillance. FluNet. www.who.int/csr/disease/
9. Molecular expressions. Cell biology and microscopy. Structure and function of cells and viruses. Optical Microscopy Division of the National High Magnetic Field Laboratory. http://micro.magnet.fsu.edu/
10. Hannoun C. Plans against influenza pandemics in Europe: history and principles. Eurosurveillance. 1998;3(3):90,191-193.
11. Miller MA, Viboud C, Balinska M, et al. The signature features of influenza pandemics--implications for policy. N Engl J Med. 2009:360(25):2595-2598.
12. Dawood FS, Jain S, Finelli L, et al. Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009:360(25):2605-2615.
13. Shinde V, Bridges CB, Uyeki T, et al. Triple-reassortant swine influenza A (H1N1) in humans in United States, 2005-2009. N Engl J Med. 2009;360(25):2616-2625.
14. Trifonov V, Khiabanian H, Greenbaum B. The origin of recent swine influenza A (H1N1) virus infecting humans. Eurosurveillance. 2009;14(17):191-193.
15. Centers for Disease Control and Prevention: Novel H1N1 vaccination recommendations. www.cdc.gov/h1n1flu/
16. Centers for Disease Control and Prevention. H1N1 flu clinical and public health guidance. www.cdc.gov/h1n1flu/guidance. Accessed August 3, 2009.
17. Musashi-Murayama, et al. Antigenic and genetic characteristics of H5N1 viruses and candidate vaccine viruses developed for potential use in human vaccines, February 2009. Wkly Epidemiol Rec. 2009;84(9):72-76.
18. Jefferson T, Pietrantonj CD, Debalini MG. Relationship of study quality, concordance, take home message, funding, and impact in studies of influenza vaccines: systematic review. BMJ.
19. Doherty PC, Kelso A. Toward a broadly protective influenza vaccine. J Clin Invest.
20. Salzberg S. The contents of the syringe. Nature. 2008;454(7201):160-161.
21. Brown EL, Kelso A. Prospects for an influenza vaccine that induces cross-protective cytotoxic T lymphocytes. Immunol Cell Biol. 2009;87:300-308.
22. Grebe KM, Hickman HD, et al. Sympathetic nervous system control of anti-influenza CD8+ T cell responses. Proc Nat Acad Sci. 2009;106(13):5300-5305. 2008;2(6):251-260. 2009;81:908-914.
23. Baras B, Bouveret N, Devaster JM, et al. A vaccine manufacturer's approach to address medical needs related to seasonal and pandemic influenza viruses. Influenza & Other Respiratory Viruses.
24. Cools HJ, Gussekloo J, Remmerswaal JE, et al. Benefits of increasing the dose of influenza vaccine in residents of long-term care facilities: a randomized placebo-controlled trial. J Med Virol.
25. Mayrhofer J, Coulibaly S, et al. Nonreplicating vaccinia virus vectors expressing the H5 influenza virus hemagglutinin produced in modified Vero cells induce robust protection. J Virol.
26. Rimmelzwaan GF, McElhaney JE. Correlates of protection: novel generation of influenza vaccines. Vaccine. 2008;36(S):D41-D44.
27. Leroux-Roels G, Leroux-Roels I. Current status and progress of prepandemic and pandemic influenza vaccine development. Expert Rev Vaccines. 2009;8(4):401-423.
28. De Filette M, Martens W, Roos K, et al. An influenza A vaccine based on tetrameric ectodomain of matrix protein 2. J Biol Chem. 2008;383(17):11382-11387.
To comment on this article, contact rdavidson@jobson.com.





