US Pharm. 2010;35(8):28-42.
In the United States, pharmacists have evolved from being solely advocates of influenza immunization to also being providers of vaccines in all 50 states. Owing to the increasing complexity of influenza and its management--including emerging strains, public concern about pandemics, widespread misconceptions about influenza vaccination, and mounting resistance to antivirals--it is crucial for pharmacists to stay abreast of emerging literature.
DESCRIPTION OF INFLUENZA
There are three influenza types (A, B, and C), of which A and B are implicated in epidemic human disease. The first portion of the influenza naming convention includes the type; next, the location of initial isolation is noted, followed by a strain designation and the year of isolation.1 For example, influenza “A/Puerto Rico/8/34” describes an influenza A virus that was first isolated in Puerto Rico in 1934.
Influenza viruses are subcategorized based on surface antigen subtypes.1 Influenza is an enveloped virus with a lipid layer to which two glycoproteins (surface antigens) are attached: hemagglutinin (HA), which mediates entry of the virus into the host cell, and neuraminidase (NA), which cleaves and releases newly formed viral particles (virions). Categorization of these structures forms the second part of an influenza viral name (e.g., H1N1). There are 16 influenza H subtypes and nine N subtypes; these form various combinations that differ in terms of genetic makeup, structure, affected hosts, and clinical manifestations. A complete description of an influenza strain includes the glycoprotein subtype--e.g., A/Fujian/411/2002 (H3N2). Sometimes influenza strains are characterized by their original host; e.g., the 2009 H1N1 strain was labeled as “swine flu” and the H5N1 strain was called “bird flu.”
HISTORY
Influenza has led to recurrent pandemics and epidemics every 1 to 3 years over the past 4 centuries. The greatest known pandemic is the 1918 influenza pandemic (nicknamed “the Spanish flu”), which infected 500 million people and killed 20 to 40 million people worldwide.2 In 2 years' time, the Spanish flu reached pandemic levels, with 20% of the world's population infected, including 28% of all Americans.
The influenza virus rapidly mutates, particularly by changing HA and NA glycoprotein subtypes, thereby undermining primary mechanisms of immunity. The progressive accumulation of mutations over time is called antigenic drift, which leads to decreasing immunity to the drifting virus. This mechanism explains the occurrence of repeated influenza epidemics and is the reason for an annual need for revaccination.3 Pandemics, on the other hand, are caused by novel influenza strains with glycoprotein subtypes unrelated to previous strains. Therefore, antibody-mediated immunity from previous influenza exposures provides no protection against the new strain. This phenomenon is known as antigenic shift. In addition, to be characterized as a pandemic, an outbreak must transcend a specific geographic area.3 Although 2009 influenza A (H1N1) was not a new subtype, few people had pre-existing antibodies to it, which resulted in widespread infection.4
EPIDEMIOLOGY
The Centers for Disease Control and Prevention (CDC) and the National Respiratory and Enteric Virus Surveillance System collaborate with laboratories across the country to determine epidemiologic trends. These findings, chronicled weekly in the CDC's FluView report, can guide practitioners in choosing appropriate empiric antiviral agents.5 In terms of cumulative epidemiologic data in the U.S., since September 1, 2009 the CDC has documented two seasonal influenza A H1N1, 14 influenza A (H3N2), 24 influenza B, and 1,814 2009 influenza A (H1N1) viruses.5 However, clinicians should keep in mind that these samples may not be representative of all patients with influenza, partly because most people manage their infections with self-care and therefore do not contribute samples.
Seasonal influenza causes annual epidemics, which typically peak in late fall through spring.6 In April 2009, a novel influenza A (H1N1) strain similar to a virus previously identified in swine began to spread across North America. This strain did not follow typical annual influenza patterns.
From 1990 to 1999, the average number of influenza-associated deaths in the U.S. was 36,000 annually, with 90% of these occurring in the elderly.7 Mortality rates have steadily increased with the aging of the population. Among children aged less than 5 years, influenza is a common cause of practitioner and ED visits, with an overall visit rate of 6% to 29% in recent years.6 Generally, influenza-associated deaths are uncommon in children. However, with the preponderance of 2009 virus, mortality in children has increased. Since the end of August 2009, there have been 273 reports of influenza-associated pediatric deaths during the influenza season.5
MANIFESTATIONS
Although influenza presentation varies, typical constitutional symptoms include fever, chills, body aches, and fatigue, while respiratory symptoms include congestion, rhinitis, sore throat, and cough.6 Differentiation between influenza and upper respiratory infections of other etiologies is challenging. Additional complaints associated particularly with 2009 influenza A (H1N1) infection are vomiting and diarrhea.6 An influenza patient is considered contagious 1 day prior to the onset of symptoms, with a peak on day 2 or 3, and for a duration of 1 week or longer. Viral shedding occurs via direct transmission (e.g., sneezing into another person's nose or mouth), the airborne route (via production and movement of aerosolized particles), or direct contact via hands or mouth. Influenza is well known as a gateway to pneumonia, which is a life-threatening complication.6 Complications are more likely in young children, the elderly, and people with underlying cardiopulmonary or other chronic diseases; for this reason, vaccination is critical in these high-risk populations.6
VACCINATION
Influenza vaccination is recognized as the most important means of preventing influenza. Effectiveness varies depending on patient factors (age, immunocompetence) and the degree of similarity between the vaccine and the circulating virus. When the influenza vaccine and the circulating virus are antigenically similar, vaccination prevents laboratory-confirmed influenza in 70% to 90% of healthy adults under the age of 65 years.8
The vaccine is trivalent, which means that it contains three strains (two influenza A, one influenza B) of different subtypes that change annually based on global surveillance for the emergence of antigenic drift. The two forms available on the market--intramuscular trivalent inactivated vaccine (TIV) and intranasal live attenuated influenza vaccine (LAIV)--have numerous key differences (TABLE 1). Most notably, TIV contains a killed virus (and therefore cannot cause influenza) and LAIV contains a live, attenuated virus (which may cause mild constitutional and respiratory symptoms such as fever and runny nose). A common and dangerous misconception, even among health care workers, is that TIV causes influenza and respiratory infection.9 The proposed 2010-2011 North American influenza vaccine will include a strain similar to the 2009 H1N1 monovalent vaccine, so it is anticipated that only a TIV will be necessary for the 2010-2011 season.5 A high-dose TIV containing four times the amount of antigen also will be available for adults aged 65 years and older.5 While this high-dose vaccine has demonstrated an improved immune response, it is unclear whether the vaccine is more effective, and therefore it is not preferred.5
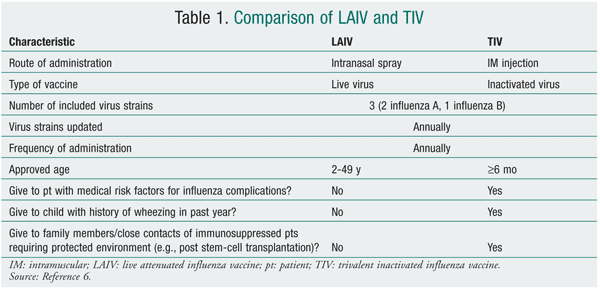
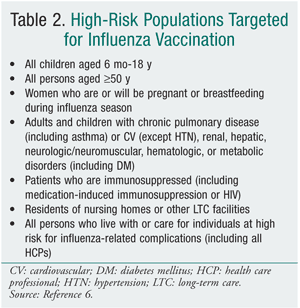
TABLE 2 lists the populations in whom vaccination is strongly recommended, although the 2010-2011 recommendations are anticipated to encourage vaccination in everyone over 6 months of age. Both vaccines are contraindicated in individuals who have anaphylactic hypersensitivity to eggs or other components of the influenza vaccine. Individuals with an acute febrile illness should wait until symptoms have subsided. A history of Guillain-Barré syndrome (GBS) within 6 weeks after a previous influenza vaccination is considered a precaution.6 The 1976 swine flu vaccination was associated with an increased frequency of GBS of 1 additional case per 100,000 persons vaccinated.10,11 The CDC, however, has stated that there is no evidence of an increase in GBS-related fatality associated with influenza vaccination.6
DRUG THERAPY
Antiviral medications are adjunctive agents for the prevention of influenza. They also are used early in the course of uncomplicated influenza infection to reduce the duration of illness by approximately 1 day.12 Adamantane derivatives (amantadine, rimantadine) and neuraminidase inhibitors (zanamivir, oseltamivir) are the two classes of agents available in the U.S. with activity against influenza (TABLES 3 and 4). Antiviral medications may inhibit replication of live virus vaccine. Because of this potential interference, LAIV should not be given 48 hours after administration of these agents and antivirals should not be given for 2 weeks after LAIV, unless medically indicated.12
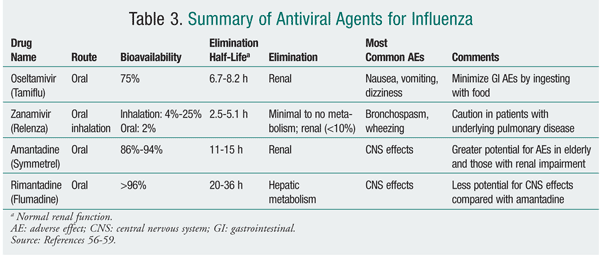
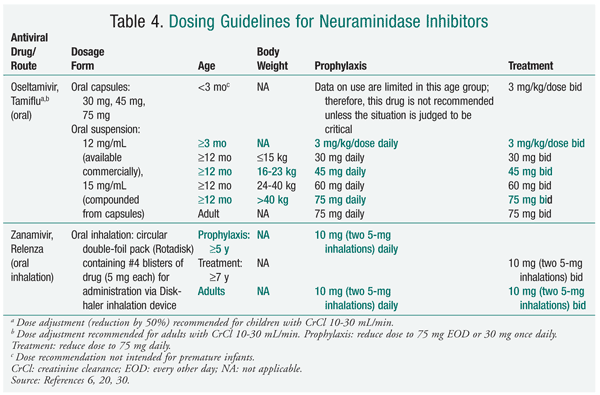
During the 2009-2010 influenza season, neuraminidase inhibitors were the preferred agents for treatment and prophylaxis. The predominance of influenza caused by 2009 influenza A (H1N1), which is resistant to adamantanes, dictated these recommendations. It is anticipated that neuraminidase inhibitors will be the mainstay for the 2010-2011 season; for this reason, this review will discuss the neuraminidase inhibitors. It is imperative that pharmacists access current CDC information to stay abreast of the most common influenza strains encountered during the influenza season, these strains' susceptibility to antiviral agents, and the most recent recommendations for treatment and prophylaxis.13
Neuraminidase Inhibitors
The neuraminidase inhibitors zanamivir and oseltamivir have activity against influenza A and B. Neuraminidase is a surface protein that is essential for the release of virus from infected cells, viral aggregation, and spreading within the respiratory tract. Both zanamivir and oseltamivir are competitive inhibitors of neuraminidase, which is expressed by influenza A and B.14 Resistance to zanamivir and oseltamivir results from mutations in viral hemagglutinin and/or neuraminidase. Mutations affecting hemagglutinin result in hemagglutinin that is less tightly bound to its own receptors, leaving the virus less dependent on neuraminidase for release from infected cells.14
The CDC tests for viral resistance to neuraminidase inhibitors. The majority of 2009 influenza A (H1N1) viruses are susceptible to oseltamivir, but rare sporadic cases of oseltamivir-resistant 2009 influenza A (H1N1) viruses have been detected. Overall, oseltamivir resistance remains under 2%. Interestingly, all tested viruses retained susceptibility to zanamivir.5
Zanamivir has a low oral bioavailability; as such, it is currently administered by oral inhalation via a disk inhaler delivery system (TABLES 3 and 4). Following administration in healthy volunteers, drug deposition was noted primarily in the oropharynx (78%) and the lung (13%-15%).15 Administration of zanamivir is dependent on activation of the device by the patient. Owing to the potential for a decline in lung function, the risks and benefits of zanamivir therapy must be carefully considered in patients with underlying diseases such as asthma or chronic obstructive pulmonary disease.16 Zanamivir powder should not be solubilized for administration via nebulizer or administered to patients on mechanical ventilation, as a death has been reported from obstruction of a ventilator circuit by lactose sugar in the formulation.14
Oseltamivir is administered orally as a prodrug, oseltamivir phosphate, which is rapidly converted by hepatic esterases to the active metabolite oseltamivir carboxylate.18 Enteric absorption of oseltamivir appears adequate in ICU patients.19 Oseltamivir carboxylate is eliminated almost entirely (>99%) by the kidney, and dose adjustments are necessary in patients with creatinine clearance <30 mL/min.20 In general, oseltamivir is well tolerated, with the most common adverse effects (AEs) being nausea and vomiting.20 Doses as high as 450 mg twice daily for 5 days in healthy volunteers were found to cause no accumulation of oseltamivir or its active metabolite; headache, nausea, vomiting, and dizziness were the most common AEs reported, the last three being dose-related.21
An increased incidence of neuropsychiatric events in influenza patients who were prescribed oseltamivir has been noted.22 Most events were more commonly reported in children early in the course of illness and close to the time that therapy was initiated. Rarely, an association with zanamivir has been reported.16 A recent review highlighted the potential for central nervous system sequelae (e.g., delirium, encephalitis) from influenza itself, so the true contribution of neuraminidase inhibitors to neuropsychiatric effects remains unclear.23
No clinically significant drug interactions are expected with zanamivir. Based on its pharmacokinetic profile, oseltamivir has a low potential for drug interactions. Probenecid, a potent competitive inhibitor of the renal tubular secretion of weak organic acids, has been shown to decrease the oseltamivir carboxylate volume of distribution by 40% and renal elimination by 61%, with a median increase in AUC of 154%.24 An in vitro study has shown that, in the presence of the antiplatelet medication clopidogrel, hydrolysis of oseltamivir was inhibited by as much as 90%.25 Since hydrolysis is required for oseltamivir's therapeutic activity, the concurrent use of these medications may decrease the therapeutic effect of oseltamivir, but the extent to which this occurs in vivo remains to be established.
Adamantane Derivatives
The adamantine derivatives amantadine and rimantadine have activity only against influenza A. These agents block the essential M2 protein, an acid-activated ion channel found only in influenza A, resulting in inhibition of viral uncoating and replication. Resistance, which can emerge spontaneously or rapidly during treatment, is conferred upon both amantadine and rimantadine.26 The CDC tests for resistance to amantadine and rimantadine.5 No resistance was noted in the seasonal influenza A (H1N1) strain, but resistance is almost complete in influenza A (H3N2) and in 2009 influenza A (H1N1).5 Therefore, the adamantanes are not reliable and are not routinely recommended for treatment of or prophylaxis against influenza.5 Pertinent characteristics of the adamantanes are summarized in TABLE 3.
Special Populations
Pregnancy and Lactation: Pregnant women are at increased risk for severe disease and complications--including death--from influenza infection.27,28 Pregnant women accounted for 5% of deaths from 2009 influenza A (H1N1) during the first 8 months of the pandemic, and influenza was more common in the second and third trimesters.27 In addition, women in their third trimester presented with more severe illness, with 49% requiring ICU admission.27 In 2009, the CDC recommended that pregnant women with influenza A (H1N1) be treated with antivirals.6 There are limited safety data, however, on the use of neuraminidase inhibitors and adamantanes in pregnancy; therefore, all of the currently available agents are designated pregnancy category C.
Embryo-fetal development studies of oseltamivir in animals reported a dose-dependent increase in the incidence of minor skeletal abnormalities in exposed offspring.20 In postmarketing surveillance, exposure to oseltamivir during pregnancy has resulted in a low rate of malformations that is within the incidence of major malformations in the general population (1%-3%).29 It is unknown to what extent oseltamivir is excreted in breast milk in humans.20
In animal studies, zanamivir crosses the placenta. Embryo-fetal development studies of IV zanamivir in animals using 300 times the exposure in humans reported no malformations, maternal toxicity, or embryotoxicity.16 During clinical trials, three pregnant women were exposed to zanamivir. One patient had a miscarriage, one pregnancy was terminated, and the third patient had a healthy birth.30 Zanamivir is excreted in breast milk in rats, but no data are available in humans.
Amantadine is teratogenic and embryotoxic in animal studies.31,32 Two case reports have identified defects in infants born to women exposed to amantadine during the first trimester.33,34 Amantadine is excreted in breast milk at low concentrations, and its use is not recommended owing to the potential for AEs.31 In animal studies, rimantadine is associated with embryotoxicity and developmental abnormalities at doses five to 11 times the recommended human dose based on body surface area.32 No data are available regarding rimantadine concentrations in human breast milk.
Data on the safety of antivirals for influenza in pregnant and lactating women remain limited. Whereas a risk has been demonstrated mainly in animal studies, that risk must be weighed against the benefits of treatment in a population that presents with a high incidence of severe disease and mortality from influenza.
Renal Replacement Therapy: In the hospitalized severely ill patient with influenza, organ dysfunction and fluid imbalances are common. Oseltamivir, in contrast to inhaled zanamivir, requires dose adjustment in patients with renal disease (TABLE 4). The use of renal replacement therapy (RRT), like hemodialysis (HD) and continuous venovenous hemodialysis (CVVHD), contributes to drug removal and further complicates drug dosing. No official dosing recommendations for oseltamivir exist for patients receiving RRT. One study in HD patients documented significant drug removal, with serum concentrations of oseltamivir carboxylate decreasing by about 70% during a 5-hour session.35 This prompted the researchers to recommend a 30-mg dose of oseltamivir after alternate HD sessions. A recent study of children receiving HD recommended dosing after each session to avoid subsequent subtherapeutic exposure.36
Not much data exist for continuous RRT. An in vitro one-compartment model of CVV hemofiltration estimated the sieving coefficient of oseltamivir carboxylate to be close to 1, suggesting that clearance of oseltamivir carboxylate can be estimated by the ultrafiltration rate.37 While the limitations of an in vitro model must be considered, data suggested that doses of oseltamivir recommended for patients with normal renal function may be appropriate in this population.37 However, a recent report in a patient receiving CVVHD and extracorporeal membrane oxygenation observed fivefold higher Cmax and AUC values compared with values from healthy volunteers, which suggests that some dose adjustment is necessary in patients receiving CVVHD.38 Additional studies in this population are needed.
Young Children: Oseltamivir has demonstrated safety and effectiveness in children aged 1 year and older, with reductions in overall clinical illness and fever and in the incidence of otitis media.39 Oseltamivir is not approved for use in children aged under 12 months, secondary to toxicities shown in juvenile rats.40 The FDA, however, released an Emergency Use Authorization (EUA) to allow oseltamivir to be used for treatment and prophylaxis in children younger than 12 months during the 2009 influenza season, citing the uncertainty of this toxicity in humans and a different risk-benefit conclusion in light of the potential for a pandemic.40
At least three case series of oseltamivir use in children aged less than 1 year have been published, all of them suggesting reasonable safety.41-43 Oseltamivir-treated children had relatively more gastrointestinal symptoms, but use was not associated with more encephalopathy or higher mortality.43 A retrospective review of 180 infants younger than 12 months who received oseltamivir, amantadine, or rimantadine noted no difference in neurologic abnormalities between oseltamivir and the adamantanes.43 The only difference was a lower incidence of head/eye/ear/nose/throat abnormalities in infants who received oseltamivir.
IV Agents
There are currently no FDA-approved agents for IV use in severe influenza. For the 2009 influenza season, the FDA released an EUA for IV peramivir. Peramivir (BioCryst Pharmaceuticals) is a neuraminidase inhibitor with comparable in vitro activity to oseltamivir and zanamivir.44 Peramivir binds more tightly to neuraminidase and has a longer dissociation half-life.45 Peramivir has low oral bioavailability and therefore is being studied as a parenteral agent. A phase II study comparing peramivir with placebo reported a shorter duration of symptoms with peramivir, with no serious AEs.46 A few case reports have documented the use of peramivir during the 2009 influenza season with no significant AEs.47,48 Peramivir maintains some inhibitory activity against zanamivir- and oseltamivir-resistant strains, but its activity depends on the type of neuraminidase mutation.49 Resistance to peramivir has been reported.
Intravenous zanamivir is approved for the treatment of influenza in some countries, but not in the U.S. The pharmacokinetics of IV zanamivir have been described.50 Several case reports have been published on the use of IV zanamivir for the treatment of severe cases of 2009 H1N1 influenza, including oseltamivir-resistant virus.51-55 Zanamivir was well tolerated, with no reports of significant AEs.
CONCLUSION
Influenza is highly contagious and is associated with significant morbidity and mortality. Vaccination is the best means of prevention, especially in high-risk individuals. Antivirals are important for preventing disease in the unvaccinated and for the treatment of patients at high risk for complications and those who present with severe disease. Pharmacists play an important role as vaccine advocates and in the appropriate selection, dosing, and safety of antivirals in the critically ill.
REFERENCES
2006;12:15-22.
1. Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 7th ed. New York, NY: Churchill Livingstone; 2010.
2. Taubenberger JK, Morens DM. 1918 influenza: the mother of all pandemics. Emerg Infect Dis.
3. Potter CW. A history of influenza. J Appl Microbiol. 2001;91:572-579.
4. Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197-201.
5. Centers for Disease Control and Prevention. FluView.
www.cdc.gov/flu/weekly. Accessed May 12, 2010.
6. Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58:1-52.
7. Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179-186.
8. Jefferson TO, Rivetti D, Di Pietrantonj C, et al. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2007(2):CD001269.
9. Mehta M, Pastor CA, Shah B. Achieving optimal influenza vaccination rates: a survey-based study of healthcare workers in an urban hospital. J Hosp Infect. 2008;70:76-79.
10. Lasky T, Terracciano GJ, Magder L, et al. The Guillain-Barré syndrome and the 1992-1993 and 1993-1994 influenza vaccines. N Engl J Med. 1998;339:1797-1802.
11. Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976-1977. Am J Epidemiol. 1979;110:105-123. 2010;115:412-414. 1999;21:267-281.
12. Gubareva LV, Kaiser L, Hayden FG. Influenza virus neuraminidase inhibitors. Lancet. 2000;355:827-835.
13. Centers for Disease Control and Prevention. Seasonal influenza. Information for health professionals.
www.cdc.gov/flu/professionals. Accessed May 3, 2010.
14. McKimm-Breschkin JL. Resistance of influenza viruses to neuraminidase inhibitors--a review. Antiviral Res. 2000;47:1-17.
15. Cass LM, Brown J, Pickford M, et al. Pharmacoscintigraphic evaluation of lung deposition of inhaled zanamivir in healthy volunteers. Clin Pharmacokinet. 1999;36(suppl 1):21-31.
16. Relenza (zanamivir) package insert. Research Triangle Park, NC: GlaxoSmithKline; February 2008.
17. Kiatboonsri S, Kiatboonsri C, Theerawit P. Fatal respiratory events caused by zanamivir nebulization. Clin Infect Dis. 2010;50:620.
18. Davies BE. Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J Antimicrob Chemother. 2010;65(suppl 2):ii5-ii10.
19. Ariano RE, Sitar DS, Zelenitsky SA, et al. Enteric absorption and pharmacokinetics of oseltamivir in critically ill patients with pandemic (H1N1) influenza. CMAJ. 2010;182:357-363.
20. Tamiflu (oseltamivir phosphate) package insert. Nutley, NJ: Roche Laboratories, Inc; February 2010.
21. Dutkowski R, Smith JR, Davies BE. Safety and pharmacokinetics of oseltamivir at standard and high dosages. Int J Antimicrob Agents. 2010;35:461-467.
22. Toovey S, Rayner C, Prinssen E, et al. Assessment of neuropsychiatric adverse events in influenza patients treated with oseltamivir: a comprehensive review. Drug Saf. 2008;31:1097-1114.
23. Toovey S. Influenza-associated central nervous system dysfunction: a literature review. Travel Med Infect Dis. 2008;6:114-124.
24. Wattanagoon Y, Stepniewska K, Lindegardh N, et al. Pharmacokinetics of high-dose oseltamivir in healthy volunteers. Antimicrob Agents Chemother. 2009;53:945-952.
25. Shi D, Yang J, Yang D, et al. Anti-influenza prodrug oseltamivir is activated by carboxylesterase human carboxylesterase 1, and the activation is inhibited by antiplatelet agent clopidogrel. J Pharmacol Exp Ther. 2006;319:1477-1484.
26. Belshe RB, Smith MH, Hall CB, et al. Genetic basis of resistance to rimantadine emerging during treatment of influenza virus infection. J Virol. 1988;62:1508-1512.
27. Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517-1525.
28. Brown CM. Severe influenza A virus (H1N1) infection in pregnancy. Obstet Gynecol.
29. Tanaka T, Nakajima K, Murashima A, et al. Safety of neuraminidase inhibitors against novel influenza A (H1N1) in pregnant and breastfeeding women. CMAJ. 2009;181:55-58.
30. Freund B, Gravenstein S, Elliott M, Miller I. Zanamivir: a review of clinical safety. Drug Saf.
31. Symmetrel (amantadine hydrochloride) package insert. Chadds Ford, PA: Endo Pharmaceuticals Inc; January 2009.
32. Flumadine (rimantadine hydrochloride) package insert. St. Louis, MO: Forest Pharmaceuticals, Inc; October 2009.
33. Pandit PB, Chitayat D, Jefferies AL, et al. Tibial hemimelia and tetralogy of Fallot associated with first trimester exposure to amantadine. Reprod Toxicol. 1994;8:89-92.
34. Nora JJ, Nora AH, Way GL. Letter: cardiovascular maldevelopment associated with maternal exposure to amantadine. Lancet. 1975;2:607.
35. Robson R, Buttimore A, Lynn K, et al. The pharmacokinetics and tolerability of oseltamivir suspension in patients on haemodialysis and continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 2006;21:2556-2562.
36. Schreuder MF, van der Flier M, Knops NB, et al. Oseltamivir dosing in children undergoing hemodialysis. Clin Infect Dis. 2010;50:1427-1428.
37. Gruber PC, Tian Q, Gomersall CD, et al. An in vitro study of the elimination of oseltamivir carboxylate by haemofiltration. Int J Antimicrob Agents. 2007;30:95-97.
38. Lemaitre F, Luyt CE, Roullet-Renoleau F, et al. Oseltamivir carboxylate accumulation in a patient treated by haemodiafiltration and extracorporeal membrane oxygenation. Intens Care Med. 2010;36:1273-1274.
39. American Academy of Pediatrics Committee on Infectious Diseases. Antiviral therapy and prophylaxis for influenza in children. Pediatrics. 2007;119:852-860.
40. Centers for Disease Control and Prevention. Emergency Use Authorization of Tamiflu (oseltamivir).
www.cdc.gov/h1n1flu/eua/
41. Okamoto S, Kamiya I, Kishida K, et al. Experience with oseltamivir for infants younger than 1 year old in Japan. Pediatr Infect Dis J. 2005;24:575-576.
42. Siedler K, Skopnik H. Oseltamivir for treatment of influenza in infants less than one year: a retrospective analysis. Pediatr Infect Dis J. 2010;29:1-4.
43. Kimberlin DW, Shalabi M, Abzug MJ, et al. Safety of oseltamivir compared with the adamantanes in children less than 12 months of age. Pediatr Infect Dis J. 2010;29:195-198.
44. Bantia S, Parker CD, Ananth SL, et al. Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob Agents Chemother. 2001;45:1162-1167.
45. Bantia S, Arnold CS, Parker CD, et al. Anti-influenza virus activity of peramivir in mice with single intramuscular injection. Antiviral Res. 2006;69:39-45.
46. Kohno S, Kida H, Mizuguchi M, Shimada J. A double-blind, placebo-controlled study of intravenous peramivir in acute influenza patients [abstract]. Presented at: 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy/Infectious Diseases Society of America 46th annual meeting; Washington, DC; October 25-28, 2008. Abstract V-4154a.
47. Campbell AP, Jacob ST, Kuypers J, et al. Respiratory failure caused by 2009 novel influenza A/H1N1 in a hematopoietic stem-cell transplant recipient: detection of extrapulmonary H1N1 RNA and use of intravenous peramivir. Ann Intern Med. 2010;152:619-620.
48. Memoli MJ, Hrabal RJ, Hassantoufighi A, et al. Rapid selection of oseltamivir- and peramivir-resistant pandemic H1N1 virus during therapy in 2 immunocompromised hosts. Clin Infect Dis.
49. Gubareva LV, Webster RG, Hayden FG. Comparison of the activities of zanamivir, oseltamivir, and RWJ-270201 against clinical isolates of influenza virus and neuraminidase inhibitor-resistant variants. Antimicrob Agents Chemother. 2001;45:3403-3408.
50. Cass LM, Efthymiopoulos C, Bye A. Pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal administration to healthy volunteers. Clin Pharmacokinet. 1999;36(suppl 1):1-11.
51. Kidd IM, Down J, Nastouli E, et al. H1N1 pneumonitis treated with intravenous zanamivir. Lancet.
52. Dulek DE, Williams JV, Creech CB, et al. Use of intravenous zanamivir after development of oseltamivir resistance in a critically Ill immunosuppressed child infected with 2009 pandemic influenza A (H1N1) virus. Clin Infect Dis. 2010;50:1493-1496.
53. Härter G, Zimmermann O, Maier L, et al. Intravenous zanamivir for patients with pneumonitis due to pandemic (H1N1) 2009 influenza virus. Clin Infect Dis. 2010;50:1249-1251.
54. Speers DJ, Williams SH, Pinder M, et al. Oseltamivir-resistant pandemic (H1N1) 2009 influenza in a severely ill patient: the first Australian case. Med J Aust. 2010;192:166-168.
55. Gaur AH, Bagga B, Barman S, et al. Intravenous zanamivir for oseltamivir-resistant 2009 H1N1 influenza. N Engl J Med. 2010;362:88-89.
56. Hayden FG, Atmar RL, Schilling M, et al. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med. 1999;341:1336-1343.
57. Cedarbaum JM. Clinical pharmacokinetics of anti-parkinsonian drugs. Clin Pharmacokinet.
58. Wintermeyer SM, Nahata MC. Rimantadine: a clinical perspective. Ann Pharmacother.
59. Keyser LA, Karl M, Nafziger AN, Bertino JS Jr. Comparison of central nervous system adverse effects of amantadine and rimantadine used as sequential prophylaxis of influenza A in elderly nursing home patients. Arch Intern Med. 2000;160:1485-1488.
To comment on this article, contact rdavidson@uspharmacist.com.





