US Pharm. 2022;47(5):43-48.
ABSTRACT: Insomnia is a common condition that can have negative effects on cognition, productivity, and quality of life. A number of different types of insomnia have been identified. Recent studies suggest that insomnia is a risk factor for mood disorders, hypertension, and relapse of depression and alcoholism. Cognitive-behavioral therapy is considered the first-line treatment for insomnia. Pharmacotherapy involves the use of sedative-hypnotics, with traditional targets of these agents being gamma-aminobutyric acid, histamine, and melatonin receptors. Alternative medications, such as antidepressants and antipsychotics, may be used in patients with certain comorbidities. Herbal supplements also have been used to treat insomnia. Orexin receptor antagonists are a novel class of sedative-hypnotics for sleep-onset and sleep-maintenance insomnia, and currently three agents have been approved by the FDA.
Most adults need about 7 to 9 hours of sleep per day.1 Insomnia, a common sleep disorder characterized by difficulty falling asleep or maintaining sleep, affects from 10% to 50% of the population worldwide.2 Insomnia is associated with adverse effects on cognition, productivity, and quality of life, and recent studies suggest that it is a risk factor for mood disorders, hypertension, and relapses of depression and alcoholism. Patients with insomnia are more likely to be absent from work and are at increased risk for occupational and motor-vehicle accidents.3
Insomnia is defined as difficulty initiating or maintaining sleep that results from daytime circumstances and is not attributable to environmental factors or inadequate opportunity to sleep.4 Different types of insomnia have been identified. Transient insomnia has a duration of less than 1 week and is caused by acute situational or environmental stressors, such as sleeping in a hotel room. Short-term insomnia has a duration of less than 3 months and is related to personal stressors, such as moving into a new house or the death of a spouse. Chronic insomnia, in which symptoms last longer than 3 months, is related to psychiatric illness, substance-abuse disorders, medical issues, or behavioral factors. Insomnia may be further divided into sleep-onset and sleep-maintenance insomnia. Sleep-onset insomnia is defined as difficulty initiating sleep, and sleep-maintenance insomnia refers to difficulty staying asleep through the night.
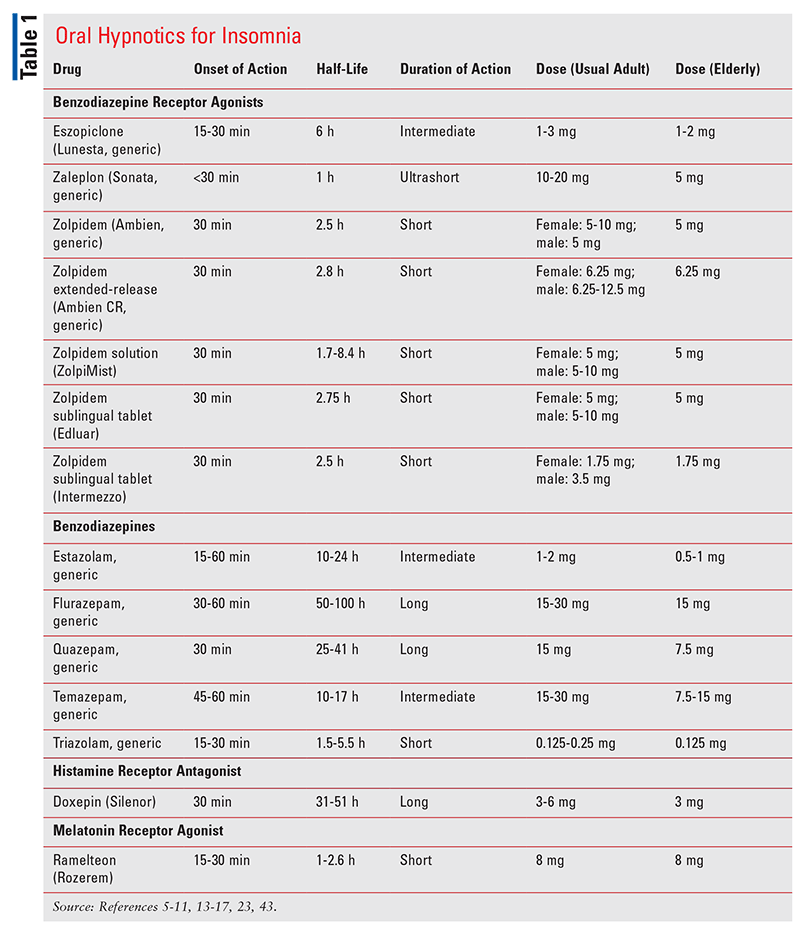
Cognitive-behavioral therapy (CBT) is the first-line treatment for insomnia.2 Pharmacotherapy for insomnia should be considered mainly in patients who are unable to participate in CBT for insomnia (CBT-I) or as an adjunct to CBT-I. Medications for insomnia have historically targeted gamma-aminobutyric acid (GABA), histamine, and melatonin receptors (TABLE 1). The newest class of medications works by antagonizing orexin receptors. The purpose of this article is to update the pharmacist on guideline-directed pharmacotherapy for insomnia and to characterize the role of orexin receptor antagonists in treatment.
PHARMACOLOGIC TARGETS
Benzodiazepine Receptor Antagonists
Members of this class include eszopiclone, zaleplon, and zolpidem.5-10 Although they are not structurally benzodiazepines, these agents bind to benzodiazepine receptors, resulting in an agonist effect on GABA. These agents decrease sleep latency (the time it takes an individual to fall asleep after turning off the lights) and are approved for short-term treatment of sleep-onset insomnia.
Eszopiclone and controlled-release zolpidem are approved for sleep-maintenance insomnia.5,8 Zolpidem is available in several dosage forms, and the sublingual tablet (Intermezzo) is approved for middle-of-the-night awakening.11 Benzodiazepine receptor agonists can cause drowsiness and dizziness; they have an additive effect with other central nervous system (CNS) depressants, and complex sleep-related behaviors have been reported. Patients who are receiving eszopiclone may experience dysgeusia (altered taste). Rifampin can induce the metabolism of benzodiazepine receptor agonists. Eszopiclone is subject to interaction with CYP3A4 inhibitors and inducers, cimetidine may inhibit zaleplon metabolism, and potent CYP3A4 inhibitors may decrease zolpidem metabolism.
Benzodiazepines
Drugs of the benzodiazepine class bind to benzodiazepine receptors on the postsynaptic GABA neuron at several sites in the CNS, thereby enhancing GABA’s inhibitive effect.12 Estazolam, flurazepam, temazepam, triazolam, and quazepam are hypnotic drugs, which are primarily used to induce and maintain sleep.13-17 Benzodiazepines reduce sleep latency but differ in onset and action. Tolerance and physical dependence develop rapidly, so these agents are not recommended for long-term use. Complex sleep-related behaviors have been reported. The Beer’s criteria, a guideline formulated by the American Geriatrics Society to reduce drug-related problems in elderly patients, does not recommend benzodiazepines.18 Triazolam is used for sleep-onset problems, and temazepam and estazolam address early-morning awakenings. The long-acting agents (flurazepam and quazepam) are for patients with sleep-maintenance problems. Benzodiazepines should be used with caution in patients with hypercapnia (commonly occurring with chronic obstructive pulmonary disease) and avoided in patients with sleep apnea. Anterograde amnesia has been reported with triazolam. The long-acting agents can impair next-day performance of activities such as driving, and they pose the risk of fatal overdose when mixed with alcohol or opioids.19 Safer alternatives are available.
Histamine Receptor Antagonists
Histamine is a stimulatory neurotransmitter that promotes wakefulness by activating H1 receptors in the cerebral cortex.20 First-generation antihistamines, such as diphenhydramine, are highly sedating owing to their effects on H1 receptors. The OTC medications diphenhydramine and doxylamine are widely used for insomnia because they are easily accessible to patients. Prescription medications (e.g., low-dose doxepin) have been identified for use in insomnia treatment because of their antihistamine affinity.
OTC sleep aids such as diphenhydramine and doxylamine are not recommended for treatment of chronic insomnia.3 These antihistamines can cause CNS depression, resulting in sedation as well as anticholinergic effects. Tolerance to diphenhydramine can develop if the drug is taken continuously.21 Diphenhydramine can cause a significant “hangover” effect, as verified by positron emission tomography.22 Next-day functioning is a significant concern for older patients who self-medicate with these agents. Patients are often unaware that diphenhydramine is an ingredient of multiple combination products in PM formulations (e.g., acetaminophen/diphenhydramine). Drug interactions include an additive anticholinergic effect or sedation with other CNS depressants. Doxylamine is contraindicated in patients younger than 12 years.
Low-dose doxepin (Silenor) is approved for treatment of sleep-maintenance insomnia. Doxepin (Sinequan), an antidepressant, is used off-label for insomnia at a dosage of 150 mg to 300 mg taken at bedtime. At a lower dose (3-6 mg), this H1 antagonist has hypnotic effects without causing significant anticholinergic effects. Doxepin should not be taken within 3 hours of a meal. Concurrent use of cimetidine (inhibitor of CYP2C19, CYP2D6, CYP1A2) should be avoided.23
Melatonin Receptor Agonists
Two melatonin receptor agonists are available: ramelteon and tasimelteon. Ramelteon is indicated for treatment of sleep-onset insomnia. Ramelteon has agonist activity at the melatonin MT1 and MT2 receptors. Agonism of melatonin receptors has a primary effect of decreasing sleep latency. Ramelteon is not limited to short-term use, and it is not a controlled substance. Comparative trials with other treatments are lacking. Ramelteon has no potential for abuse, but its overall hypnotic effect is limited.24 No rebound insomnia or withdrawal has been reported. Ramelteon should be used cautiously with concurrent use of CYP1A2 inhibitors such as ciprofloxacin or CYP3A4 inhibitors such as fluconazole. Rifampin is a potent inducer of ramelteon metabolism. Adverse reactions of ramelteon include complex sleep behaviors, increased prolactin, and decreased serum testosterone.25
Tasimelteon (Hetlioz) is indicated for treatment of non-24 sleep-wake disorder (non-24). Non-24 is a chronic circadian-rhythm disorder in blind individuals that affects the timing of sleep. Because in blind patients light does not enter the eyes, the body cannot synchronize to the 24-hour light-dark cycle. The dosage is 20 mg given 1 hour prior to bedtime, at the same time each evening. Strong CYP3A4 inhibitors (fluvoxamine) and inducers (rifampin) should be avoided.26
Alternative Sleep Medications
In patients with comorbidities such as depression, seizure disorder, or psychotic disorders, insomnia may be treated with antidepressants or antipsychotics.3 Examples of these alternative medications for sleep disorders include alprazolam, amitriptyline, clonazepam, clonidine, trazodone, mirtazapine, and quetiapine. There is little evidence supporting the use of these treatments for insomnia that is not associated with a comorbid condition. Quetiapine is the most commonly used antipsychotic for sleep disorders. Interestingly, trazodone is one of the most widely used sedative agents, yet has the least amount of evidence.27 For mirtazapine to be used as a sedative-hypnotic, it must be dosed at 7.5 mg to 15 mg at bedtime, which affords the most affinity for anticholinergic effects.28
Orexin Receptor Antagonists
Orexins (formerly known as hypocretins) are endogenous neuropeptides that activate orexin receptors 1 and 2 in the lateral hypothalamic region of the brain.29 Orexin receptor stimulation is primarily excitatory and releases a variety of neurotransmitters that are responsible for the maintenance of arousal, wakefulness, and appetite. Currently, three orexin receptor antagonists—suvorexant, lemborexant, and daridorexant—are FDA approved for the treatment of insomnia (TABLE 2).30-32 Several orexin receptor antagonists are being investigated for insomnia in Alzheimer’s disease, appetite suppression in obesity, and a number of mental-health conditions, including depression, drug dependence, panic disorder, and posttraumatic stress disorder.33
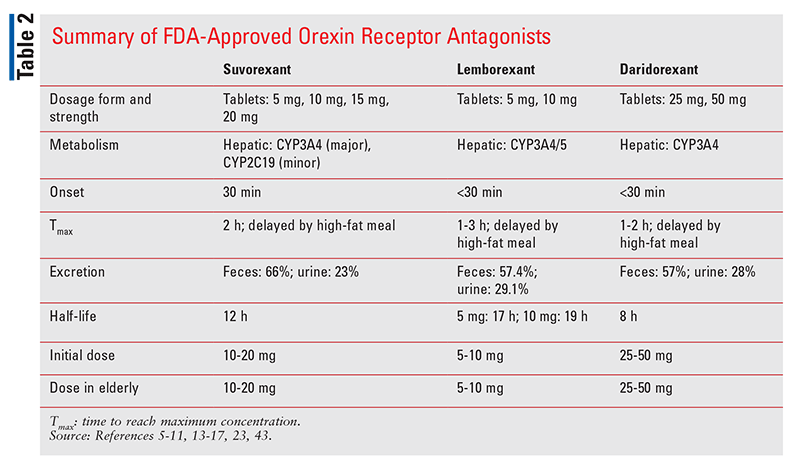
Suvorexant (Belsomra): Suvorexant is a nonselective orexin receptor antagonist approved in 2014 for the treatment of both sleep-onset and sleep-maintenance insomnia.30 Approval was based on data from three double-blind, placebo-controlled studies.30,33 Suvorexant achieves peak plasma concentrations in a median of 2 hours (range: 30 minutes to 6 hours), but ingestion with food results in a delay. This agent has an onset of action of 30 minutes and a half-life of 12 hours.30 It is primarily metabolized by CYP3A4, with a minimal contribution from CYP2C19. Suvorexant is available in 5-, 10-, 15-, and 20-mg tablets. The starting dosage of suvorexant is 10 mg taken within 30 minutes before bedtime, with at least 7 hours remaining prior to planned awakening. If 10-mg dosing is not effective, the dosage may be increased to a maximum of 20 mg once daily. Dosing cannot be repeated if induction of sleep is not achieved or if sleep maintenance fails. In patients receiving moderate CYP3A4 inhibitor therapy, the initial dosage is 5 mg once daily, and this may be increased to a maximum of 10 mg once daily if the 5-mg dose is not effective. Suvorexant should not be used concurrently with strong CYP3A4 inhibitors. CYP3A4 inducers may reduce the effectiveness of suvorexant.30
Lemborexant (Dayvigo): Lemborexant, which was approved for the treatment of insomnia in 2019, is a more selective orexin-2 receptor antagonist compared with suvorexant.31 The SUNRISE-1 trial compared lemborexant with extended-release zolpidem and placebo, and the SUNRISE-2 trial compared lemborexant with placebo.31,34 Lemborexant’s time to achieve peak plasma concentration is 1 to 3 hours, although administration with food causes a delay. This agent has a half-life of 17 to 19 hours and is primarily metabolized by CYP3A4.35 Lemborexant is available as 5- and 10-mg tablets, and the recommended starting dosage is 5 mg immediately before going to bed, with an intended sleep duration of 7 hours. The dosage may be increased to a maximum of 10 mg once daily if the 5-mg dose is not effective. In patients taking weak CYP3A4 inhibitors, the maximum lemborexant dosage is 5 mg once daily. Lemborexant is not recommended in patients receiving moderate to strong CYP3A4 inhibitors or CYP3A4 inducers.35
Daridorexant (Quviviq): This small-molecule dual orexin 1 and 2 receptor antagonist was approved by the FDA in January 2022.32,36 Its expected availability is May 2022, following scheduling by the U.S. Drug Enforcement Administration. Approval was based on two phase III placebo-controlled trials in 1,854 adults with insomnia.32 The primary end points, sleep latency and sleep maintenance, were significantly improved versus placebo at 3-month follow-up. Extension studies of these two trials demonstrated continued efficacy for up to 12 months. The approved dosage of daridorexant is 25 mg or 50 mg taken once nightly 30 minutes before bedtime, with at least 7 hours of planned sleep. Daridorexant is a Schedule IV controlled substance.32
Effects of Orexin Receptor Antagonists: The most common adverse effects from orexin receptor antagonists are somnolence and fatigue. Warnings common to all hypnotics that have been reported with orexin receptor antagonists include CNS depression and daytime somnolence.30,31 These effects are dose related. Other adverse effects include the occurrence of complex sleep behaviors (e.g., sleepwalking, sleep driving), sleep paralysis, hypnagogic/hypnopompic hallucinations, and worsening of depression or suicidal ideation.37 Suvorexant, lemborexant, and daridorexant are contraindicated in patients with narcolepsy, and they are not recommended in patients with severe hepatic dysfunction.
Compared with placebo, all three orexin receptor antagonists have shown beneficial effects in total sleep time, decreases in waking after sleep onset, and subjective sleep latency.37,38 Although head-to-head randomized comparisons of hypnotics are lacking, orexin receptor antagonists do not appear to be associated with substantial respiratory depression. Orexin receptor antagonists seem to be generally well tolerated in elderly patients. There is no evidence of addiction potential, and these agents do not appear to produce dependency or tolerance-inducing effects.37,38 Orexin receptor antagonists seem to improve rapid eye movement sleep, which may be an advantage in patients with Alzheimer’s disease.37 Orexin receptor antagonists have additional potential advantages over benzodiazepines and other hypnotics, including limited cognitive impairment and the ability to quickly arouse patients from sleep with adequate stimulation.37,38 One potential major limitation to the use of orexin receptor antagonists is their cost, which typically exceeds $10 per day without prescription insurance coverage.
Traditional Herbal Remedies
Some dietary supplements are marketed as sleep aids. Some examples are melatonin, valerian root, German chamomile, kava, L-tryptophan, littleleaf linden (Tilia), vervain (verbena), melissa (lemon balm), and skullcap (Scutellaria). These products are not regulated, and the purity of ingredients and clinical response can vary. There is a paucity of evidence supporting the use of these herbal remedies, and although most are considered safe, data on drug interactions with prescription medications are lacking.3 Melatonin, the most widely used supplement for insomnia, is available in multiple dosage forms and may be beneficial when taken episodically; it is not recommended for chronic insomnia, however. For optimal performance, melatonin is dosed at 3 mg to 5 mg and must be taken a few hours before bedtime. The long-term safety of melatonin is not known.39
THE PHARMACIST’S ROLE
Pharmacists should be familiar with orexin receptor antagonists and the pharmacologic profiles of all sedative-hypnotics. Agents vary by onset of action, half-life, duration of effect, clinical application, and dosage range. Most sedative-hypnotics are Schedule IV medications, exceptions being doxepin and ramelteon. Pharmacists should monitor concurrent prescription and nonprescription medications, and the combining of hypnotics with alcohol and other CNS depressants should be avoided. Hypnotics should be used with caution in older adults, and screening for drug interactions is critical. Counseling patients on appropriate use of hypnotics and timing of dose with regard to meals and desired sleep onset is important for optimal outcomes. Adverse-effect monitoring includes assessment of risks to next-day performance, alertness, driving skills, and complex sleep behaviors. Pharmacists should be aware that insomnia is a recognized risk for suicide in patients with underlying depression, and FDA-labeled insomnia medications carry a warning or precaution about suicide risk in depressed patients.40,41
GUIDELINE RECOMMENDATIONS
The orexin receptor antagonists are not addressed in all clinical practice guidelines, as some guidelines have not been updated since the approval of these agents. The American Academy of Sleep Medicine suggests that suvorexant be used to treat sleep-maintenance insomnia (versus no treatment) in adults (weak recommendation; low quality of evidence).3 The Department of Veterans Affairs states that evidence is insufficient to recommend for or against the use of suvorexant for the treatment of chronic insomnia disorder (i.e., recommends neither for nor against).42 Lemborexant is not specifically mentioned in any sleep guideline. As noted earlier, daridorexant was only recently approved.
CONCLUSION
Sleep medications should ideally be used in conjunction with CBT-I. Treatment selection and duration for insomnia should be individualized depending on the patient’s clinical response. Pharmacologic treatment of insomnia begins with the lowest effective dose. If treatment is nightly, short-term use is best; if it is intermittent, longer-term use is acceptable. Ramelteon, eszopiclone, and controlled-release zolpidem are FDA approved for chronic use. Suvorexant and lemborexant, the first two FDA-approved orexin receptor antagonists, are effective therapies for insomnia but have not been compared head-to-head in clinical trials; the third agent, daridorexant, was recently approved and is not yet available.
REFERENCES
1. Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40-43.
2. Bhaskar S, Hemavathy D, Prasad S. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. J Family Med Prim Care. 2016;5(4):780-784.
3. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
4. Sateia MJ. International classification of sleep disorders–third edition: highlights and modifications. Chest. 2014;146(5):1387-1394.
5. Lunesta (eszopiclone) package insert. Marlborough, MA: Sunovion Pharmaceuticals Inc; May 2014.
6. Sonata (zaleplon) package insert. New York, NY: Pfizer Inc; December 2019.
7. Ambien (zolpidem tartrate) package insert. Bridgewater, NJ: sanofi-aventis U.S. LLC; February 2022.
8. Ambien CR (zolpidem tartrate extended-release) package insert. Bridgewater, NJ: sanofi-aventis; September 2020.
9. ZolpiMist (zolpidem tartrate) oral spray package insert. Englewood, CO: Aytu Bioscience, Inc; August 2019.
10. Edluar (zolpidem tartrate) package insert. Somerset, NJ: Meda Pharmaceuticals Inc; September 2021.
11. Intermezzo (zolpidem tartrate) package insert. Richmond, CA: Transcept Pharmaceuticals, Inc; November 2011.
12. Bounds CG, Nelson VL. Benzodiazepines. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022.
13. Estazolam package insert. Parsippany, NJ: Actavis Pharma, Inc; January 2021.
14. Dalmane (flurazepam) package insert. Aliso Viejo, CA: Valeant Pharmaceuticals North America; April 2007.
15. Restoril (temazepam) package insert. Hazelwood, MO: Mallinckrodt Pharmaceuticals; September 2016.
16. Halcion (triazolam) package insert. New York, NY: Pfizer Inc; October 2021.
17. Doral (quazepam) package insert. Atlanta, GA: Galt Pharmaceuticals, LLC; January 2021.
18. The 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-694.
19. Tanaka E. Toxicological interactions between alcohol and benzodiazepines. J Toxicol Clin Toxicol. 2002;40(1):69-75.
20. Szabadi E. Drugs for sleep disorders: mechanisms and therapeutic prospects. Br J Clin Pharmacol. 2006;61(6):761-766.
21. Richardson GS, Roehrs TA, Rosenthal L, et al. Tolerance to daytime sedative effects of H1 antihistamines. J Clin Psychopharmacol. 2002;22(5):511-515.
22. Zhang D, Tashiro M, Shibuya K, et al. Next-day residual sedative effect after nighttime administration of an over-the-counter antihistamine sleep aid, diphenhydramine, measured by positron emission tomography. J Clin Psychopharmacol. 2010;30(6):694-701.
23. Silenor (doxepin) package insert. Morristown, NJ: Currax Pharmaceuticals LLC; October 2020.
24. Kuriyama A, Honda M, Hayashino Y. Ramelteon for the treatment of insomnia in adults: a systematic review and meta-analysis. Sleep Med. 2014;15(4):385-392.
25. Rifadin (rifampin) package insert. Bridgewater, NJ: sanofi-aventis U.S. LLC; January 2022.
26. Hetlioz (tasimelteon) package insert. Washington, DC: Vanda Pharmaceuticals Inc; December 2020.
27. Mendelson WB. A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry. 2005;66(4):469-476.
28. Kasper S, Praschak-Rieder N, Tauscher J, Wolf R. A risk-benefit assessment of mirtazapine in the treatment of depression. Drug Saf. 1997;17(4):251-264.
29. Gotter AL, Webber AL, Coleman PJ, et al. International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol Rev. 2012;64(3):389-420.
30. Belsomra (suvorexant) package insert. Whitehouse Station, NJ: Merck & Co, Inc; March 2021.
31. Dayvigo (lemborexant) package insert. Nutley, NJ: Eisai Inc; March 2022.
32. Quviviq (daridorexant) package insert. Radnor, PA: Idorsia Pharmaceuticals US Inc; January 2022.
33. Janto K, Prichard JR, Pusalavidyasagar S. An update on dual orexin receptor antagonists and their potential role in insomnia therapeutics. J Clin Sleep Med. 2018;14(8):1399-1408.
34. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254.
35. Scott JL. Lemborexant: first approval. Drugs. 2020;80:425-432.
36. Roch C, Bergamini G, Steiner MA, Clozel M. Nonclinical pharmacology of daridorexant: a new dual orexin receptor antagonist for the treatment of insomnia. Psychopharmacology (Berl). 2021;238(10):2693-2708.
37. Hoyer D, Allen A, Jacobson LH. Hypnotics with novel modes of action. Br J Clin Pharmacol. 2020;86(2):244-249.
38. Rios P, Cardoso R, Morra D, et al. Comparative effectiveness and safety of pharmacological and non-pharmacological interventions for insomnia: an overview of reviews. Syst Rev. 2019;8(1):281.
39. Andersen LPH, Gögenur I, Rosenberg J, Reiter RJ. The safety of melatonin in humans. Clin Drug Investig. 2016;36(3):169-175.
40. Bryan CJ, Gonzales J, Rudd MD, et al. Depression mediates the relation of insomnia severity with suicide risk in three clinical samples of U.S. military personnel. Depress Anxiety. 2015;32(9):647-655.
41. McCall WV, Benca RM, Rosenquist PB, et al. Hypnotic medications and suicide: risk, mechanisms, mitigation, and the FDA. Am J Psychiatry. 2017;174(1):18-25.
42. Mysliwiec V, Martin JL, Ulmer CS, et al. The management of chronic insomnia disorder and obstructive sleep apnea: synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense clinical practice guidelines. Ann Intern Med. 2020;172(5):325-336.
43. Rozerem (ramelteon) package insert. Deerfield, IL: Takeda Pharmaceuticals America, Inc; November 2010.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





