US Pharm. 2023;48(2):18-21.
ABSTRACT: The human microbiome consists of diverse bacteria, fungi, protozoa, and viruses that can influence or be influenced by orally ingested medications. Microorganisms within the gastrointestinal tract, in particular, can activate, inactivate, or modify oral medications into toxic metabolites, leading to alterations in drug bioavailability, which could impact patient care. Pharmacomicrobiomics, a relatively new science involving interactions between the microbiota and medications for disease treatment, could be crucial for understanding the variability in patient responses to these drugs. Certain gut microbes specifically affect the pharmacokinetics of a number of common cardiovascular drugs. An exploration of the complex relationship between the gut microbiome and cardiovascular drugs will promote a path toward greater personalization of medication therapy to achieve positive health outcomes for all patients.
So small that they are invisible to the naked eye, microorganisms are present on almost every part of the human body, including the gastrointestinal tract, skin, and lungs. These microbiota are often referred to as the “invisible organ” because they are partially responsible for digestion, vitamin synthesis, defense against pathogens, and other beneficial effects. A healthy microbiota—comprising more microbial cells than the human body it inhabits—contains a wide variety of diverse bacteria, fungi, protozoa, and viruses, and its composition is shaped by every aspect of life, including geography and diet.1 Although knowledge of the microbiota that comprise the human microbiome dates back to the mid-1880s, systematic studies of microbe-host relationships were not conducted until relatively recently, following the proliferation of new genomic-analysis technologies. One of these emerging fields of study, pharmacomicrobiomics, explores the interactions between a person’s microbiota and medications for disease treatment.1
It has been demonstrated that the gut microbiome, in particular, can influence or be influenced by many commonly prescribed drugs.2 Orally administered medications travel the length of the digestive tract before reaching the intestines, where they are ultimately absorbed into systemic circulation. As shown in FIGURE 1, microorganisms within the gastrointestinal tract can activate, inactivate, or even modify oral medications into toxic metabolites, leading to alterations in drug bioavailability that could impact patient care.3-8 Commonly used medications also impact the abundance and diversity of the microbiome, consequently affecting digestion, vitamin synthesis, and more.
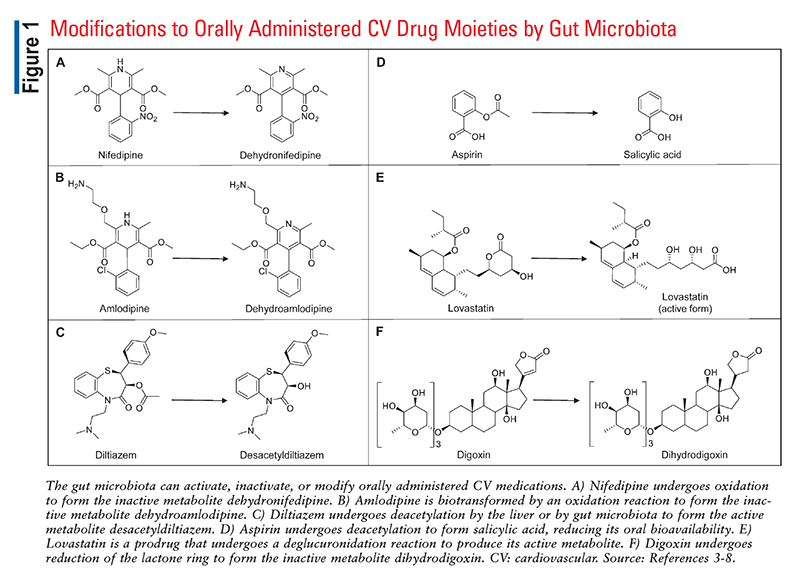
The microbiota is known to affect the pharmacokinetics of many common cardiovascular drugs. The Western diet, which is typically high in processed foods, tends to increase a patient’s risk for developing cardiovascular conditions; additionally, it is directly associated with dysbiosis, or disruption of the normal gut microbiota.9 Owing to the prevalence of the Western diet, dysbiosis, and cardiovascular disease, cardiovascular drugs have consistently been some of the most commonly dispensed medications in the United States. Therefore, knowledge of the pharmacomicrobiomics of cardiovascular drugs could be crucial for understanding the variability in patient responses to these medications.
Amlodipine and Nifedipine
Amlodipine and nifedipine are calcium channel blockers that are used to treat hypertension. Oxidation reactions by gut microorganisms biotransform these agents into inactive metabolites, which are then excreted in the feces.4 Although many patients will achieve a therapeutic effect from these medications despite the bacterial inactivation of a portion of the dose, recent clinical studies have identified some common situations that may impact the overall therapeutic approaches for these drugs.
In one study, Yoo and colleagues investigated the coadministration of amlodipine and ampicillin to determine how antibiotic therapy impacts the gut microbiome and amlodipine pharmacokinetics. When antibiotics were given with amlodipine, the gut microbiome’s biotransformation effect was suppressed and the systemic bioavailability of amlodipine was increased.4 The rate and extent of amlodipine absorption were significantly increased during coadministration with ampicillin, as the gut microbiome was impaired and less likely to deactivate the drug. This effect held true when other antibiotic classes, such as tetracyclines, macrolides, and cephalosporins, were used.4 Although the magnitude and clinical impact of this interaction are difficult to determine, it may be important to monitor patients to ensure that no supratherapeutic effects, such as hypotension, occur, given the increased bioavailability of amlodipine when administered concurrently with certain antibiotics.
Zhang and colleagues examined possible effects of altitude on hypoxia and gut-microbe diversity and activity.3 Knowing that gut microflora inactivate nifedipine, the researchers simulated a high-altitude, low-oxygen environment and monitored the number of microorganisms present as well as their bioactivity. The hypoxic environment resulted in a reduction in the number of Enterobacteriaceae, the gram-negative rod that is a normal component of the gut’s microbiome and one of the organisms responsible for this metabolic deactivation.3 The researchers hypothesized that the presence of fewer of these organisms could mean higher nifedipine bioavailability, which might have significant effects for travelers who transition rapidly from low-altitude to high-altitude environments.3
Diltiazem
Diltiazem is a nondihydropyridine calcium channel blocker that is used in the treatment of hypertension and angina. This cardiac drug undergoes hepatic metabolism by both CYP3A4 and CYP2D6 into two active metabolites, desacetyldiltiazem and desmethyldiltiazem.5 In recent research, Zimmermann and colleagues identified a bacterium in the human gut that also contains enzymes that metabolize diltiazem into these two active metabolites.5 The microorganism, Bacteroides thetaiotaomicron, may use these enzymes to deacetylate certain sugars as part of the normal digestive process, meaning that the effects on diltiazem are incidental.10 How much the gut microbiome influences diltiazem’s pharmacologic effects on blood pressure or heart rate remains unknown, but it is clear that serum concentrations of the parent drug and its less active metabolites are influenced by the metabolic capacity of B thetaiotaomicron in the gut when this organism is present.10
Aspirin
Although many people enjoy their daily cup of coffee, most are likely unaware of how coffee affects the gut microbiome, particularly in relation to aspirin. In a prospective study by Kim and colleagues, administration of coffee bean extract changed the biodiversity of gut bacteria, leading to an increase in the number of Lactobacillaceae and Muribaculaceae organisms in the gut and a decrease in Proteobacteria, Helicobacteriaceae, and Bacteroidaceae concentrations.6 The researchers also evaluated blood concentrations of aspirin in mice as well as adult men treated with a combination of coffee bean extract and aspirin. Gut bacteria hydrolyze aspirin to an ionized form that is less likely to be absorbed in the intestines, but because coffee bean extract impairs the activity of gut microbes, the coffee-aspirin combination resulted in increased concentrations of nonionized, highly bioavailable drug (the absolute increase in absorption was very small, however).6
Interestingly, and apart from its cardiovascular uses, aspirin may also confer a protective effect against colorectal cancer by modulating gut flora. Results from Prizment and colleagues’ pilot randomized, controlled trial of 50 patients demonstrated that a 6-week course of aspirin was associated with an increase in the number of Akkermansia organisms, which have been associated with improved survival and anticancer immune responses in patients with colorectal cancer.11 Aspirin also reduced the concentrations of Parabacteroides and Dorea species, which are typically increased in these patients.11
Statins
The statin drug class is known to inhibit the cholesterol-synthesizing enzyme 3-hydroxy-3-methylglutaryl coenzyme A reductase, which is present in humans and in certain bacterial organisms, such as Enterococcus faecalis and Enterococcus faecium. The interactions between statins, bile acid metabolism, cholesterol synthesis, and the gut microbiome are quite complex; therefore, they have been the target of many recent studies. Importantly, certain species of gut microbiota (including well-studied probiotic strains such as Lactobacillus and Bifidobacterium) are vital sources of a group of enzymes known as bile salt hydrolases (BSHs).12 Bile salts are synthesized from cholesterol by the liver and then metabolized by the gut microbiome into a variety of bile salt derivatives, which are then reabsorbed and further metabolized by the liver, giving rise to a large, diverse pool of complex bile salts that aid in digestion and may protect against harmful organisms such as Clostridium difficile.12 Because LDL-cholesterol concentrations are inversely correlated with circulating bile acids, it has been hypothesized that the cholesterol-lowering effect of statins may be linked to the activity of bacteria-containing BSHs such as Lactobacillus. In a randomized, placebo-controlled clinical trial involving 127 participants, treatment with the probiotic Lactobacillus reuteri was shown to significantly reduce LDL-cholesterol concentrations.13
Uniquely among the statins, lovastatin is a prodrug that must be metabolized by the gut microbiome from a gamma-lactone closed ring to the beta hydroxy acid open-ring form in order to be active.7 Yoo and colleagues investigated impairment of lovastatin bioactivation by the gut microbiome in antibiotic-treated rats given several different antibiotics, including ampicillin and a broad-spectrum antibiotic mixture consisting of cefadroxil, oxytetracycline, and erythromycin.7 The concentration of the active metabolite of lovastatin was approximately 60% lower in antibiotic-treated rats than in control rats, which were not given antibiotics.7 These results suggest that antibiotics may reduce the effectiveness of lovastatin by disrupting the gut microbiome’s bioactivation of the prodrug.
Consideration of how the microbiome directly influences lovastatin pharmacokinetics and how the statin class decreases the amount of specific bacterial species and therefore modulates the enzymes involved in bile acid metabolism makes it apparent that the most common cholesterol-lowering drugs are also impacting patients’ critical gut microbiota and their cardiovascular health.7,13 Exploration of the complex relationships existing between the gut microbiome and cardiovascular drugs, including cholesterol-lowering therapies, should continue.
Digoxin
Digoxin is a cardiac glycoside that is used to treat atrial fibrillation and heart failure. In the 1920s, scientists first recognized that some people taking digoxin excreted the inactive metabolite dihydrodigoxin, which is formed by the nonphysiologic reduction of the lactone ring.8 More recently, studies have identified a certain strain of the gut bacterium Eggerthella lenta as the only possible source of this metabolic process in vivo.8,14 An estimated 10% of digoxin patients are impacted by this phenomenon, wherein a large proportion of an orally administered digoxin dose is inactivated by the individual’s gut flora. In a study conducted by Lindenbaum and colleagues, antibiotic therapy inhibited this deactivation process, resulting in a nearly twofold increase in serum digoxin concentrations.15
In addition to determining the specific strain of E lenta responsible for this metabolic process, Haiser and colleagues found that the dietary amino acid arginine decreased digoxin inactivation.8 Arginine is essential for the growth of E lenta, and in mouse models arginine supplementation appeared to enhance the organism’s growth while simultaneously inhibiting its metabolic deactivation of digoxin.8 The researchers posited that studies of the effects of the gut microbiome might one day inform precision medicine by guiding dietary or supplement-based interventions targeting modifications to gut flora.8
Warfarin
The anticoagulant warfarin is a vitamin K antagonist well known for its narrow therapeutic index and the need for close therapeutic monitoring. Organisms that are relevant in patients taking warfarin include Escherichia coli and Shigella species, both of which appear to play a key role in the biosynthesis of menaquinone, or vitamin K2.16 Research by Wang and colleagues found a positive correlation between the amount of vitamin K in the feces and the amount of Escherichia-Shigella in an individual’s gut microbiome.2 Because having a greater number of these organisms leads to greater production of vitamin K, these patients may experience a reduced response to warfarin therapy. Conversely, an abundance of the gut microbe Enterococcus was associated with low concentrations of vitamin K in the feces and increased response to warfarin therapy.2 Because warfarin underdosing or overdosing can result in dire patient outcomes, the effects of microbiome variability on vitamin K synthesis should be further investigated as a means of assessing individual patients’ response to warfarin therapy.
Amiodarone
The antiarrhythmic agent amiodarone is often used to treat ventricular tachycardia and fibrillation; however, because of this drug’s narrow therapeutic index, organ toxicity has occurred with amiodarone overexposure. Matuskova and colleagues demonstrated that rats administered a probiotic containing a particular strain of E coli Nissle 1917 experienced plasma amiodarone concentrations up to 1.4 times higher than concentrations in control rats receiving either saline or the nonprobiotic strain of E coli.17 The exact cause of the increased bioavailability with this specific organism has not been identified, but increased drug absorption into systemic circulation is a plausible explanation. Theories for this increased absorption include 1) a decrease in local pH mediated by the microorganisms that ionizes and facilitates amiodarone’s absorption through the mucosal layer and 2) an increase in the expression of a particular cellular membrane transporter that mediates the uptake of amiodarone.17 As with warfarin, the narrow therapeutic index and adverse drug reactions of amiodarone make it crucial to better understand the influence of the microbiota on this drug.
Conclusion
Each person harbors an individually distinct gut microbiome that can be altered by many things, such as diet, environment, and cardiovascular drugs. It is becoming apparent that commonly used cardiovascular drugs interface with a person’s “invisible organ” in numerous and unpredictable ways. The studies referenced here describe early evidence that a patient’s microbiome directly influences drug pharmacokinetics, just as medications alter concentrations of the many types of human gastrointestinal microorganisms. Exploration of the complex relationship between the gut microbiome and cardiovascular drugs will promote a path toward greater personalization of medication therapy to achieve positive health outcomes for all patients.
REFERENCES
1. Rizkallah MR, Saad R, Aziz RK. The Human Microbiome Project, personalized medicine and the birth of pharmacomicrobiomics. Curr Pharmacogenomics Personalized Med. 2010;8(3):182-193.
2. Wang L, Liu L, Liu X, et al. The gut microbes, Enterococcus and Escherichia-Shigella, affect the responses of heart valve replacement patients to the anticoagulant warfarin. Pharmacol Res. 2020;159:104979.
3. Zhang J, Chen Y, Sun Y, et al. Plateau hypoxia attenuates the metabolic activity of intestinal flora to enhance the bioavailability of nifedipine. Drug Deliv. 2018;25(1):1175-1181.
4. Yoo HH, Kim IS, Yoo DH, Kim DH. Effects of orally administered antibiotics on the bioavailability of amlodipine: gut microbiota-mediated drug interaction. J Hypertens. 2016;34(1):156-162.
5. Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019;570(7762):462-467.
6. Kim JK, Choi MS, Yoo HH, Kim DH. The intake of coffee increases the absorption of aspirin in mice by modifying gut microbiome. Pharmaceutics. 2022;14(4):746.
7. Yoo DH, Kim IS, Le TKV, et al. Gut microbiota-mediated drug interactions between lovastatin and antibiotics. Drug Metab Dispos. 2014;42(9):1508-1513.
8. Haiser HJ, Seim KL, Balskus EP, Turnbaugh PJ. Mechanistic insight into digoxin inactivation by Eggerthella lenta augments our understanding of its pharmacokinetics. Gut Microbes. 2014;5(2):233-238.
9. Zinöcker MK, Lindseth IA. The Western diet-microbiome-host interaction and its role in metabolic disease. Nutrients. 2018;10(3):365.
10. Zhou S, Ko TP, Huang JW, et al. Structure of a gut microbial diltiazem-metabolizing enzyme suggests possible substrate binding mode. Biochem Biophys Res Commun. 2020;527(3):799-804.
11. Prizment AE, Staley C, Onyeaghala GC, et al. Randomised clinical study: oral aspirin 325 mg daily vs placebo alters gut microbial composition and bacterial taxa associated with colorectal cancer risk. Aliment Pharmacol Ther. 2020;52(6):976-987.
12. Foley MH, O’Flaherty S, Barrangou R, Theriot CM. Bile salt hydrolases: gatekeepers of bile acid metabolism and host-microbiome crosstalk in the gastrointestinal tract. PLoS Pathog. 2019;15(3):e1007581.
13. Jones ML, Martoni CJ, Prakash S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. Eur J Clin Nutr. 2012;66(11):1234-1241.
14. Saha JR, Butler VP Jr, Neu HC, Lindenbaum J. Digoxin-inactivating bacteria: identification in human gut flora. Science. 1983;220(4594):325-327.
15. Lindenbaum J, Rund DG, Butler VP Jr, et al. Inactivation of digoxin by the gut flora: reversal by antibiotic therapy. N Engl J Med. 1981;305(14):789-794.
16. Palaniappan C, Sharma V, Hudspeth ME, Meganathan R. Menaquinone (vitamin K2) biosynthesis: evidence that the Escherichia coli menD gene encodes both 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylic acid synthase and alpha-ketoglutarate decarboxylase activities. J Bacteriol. 1992;174(24):8111-8118.
17. Matuskova Z, Anzenbacherova E, Vecera R, et al. Administration of a probiotic can change drug pharmacokinetics: effect of E. coli Nissle 1917 on amiodarone absorption in rats. PLoS One. 2014;9(2):e87150.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





