US Pharm. 2022;47(6):37-42.
ABSTRACT: Diabetes is a chronic disease in which the body’s blood glucose concentration is abnormally high, leading to the risk of serious health problems. There are three main types of diabetes: type 1, type 2, and gestational. According to the CDC, more than 11% of the U.S. population had some type of diabetes in 2019. Insulin therapy is a vital part of diabetes management. Two insulin biosimilars for insulin glargine (Lantus), a subcutaneous long-acting insulin analogue for improving glycemic control, have recently been approved: Semglee (insulin glargine-ygfn), which is an interchangeable product, and Rezvoglar (insulin glargine-aglr), which is not interchangeable. These biosimilars provide diabetic patients with effective, affordable alternatives to insulin glargine.
Diabetes, a chronic disease in which the body’s blood glucose (BG) concentration is abnormally high, is the seventh leading cause of death in the United States.1,2 The CDC estimates that 37.3 million Americans, or 11.3% of the U.S. population, had diabetes in 2019.1 BG (also known as blood sugar) is the body’s chief source of energy and derives primarily from food. Insulin (discussed further below) aids in regulating food-acquired glucose for use as energy in the cells. When the body produces insufficient or no insulin or does not use it well, glucose cannot enter the cells and remains in the blood; this eventually leads to an increased risk of serious health problems.2
Diabetes is classified into three main types: type 1 diabetes (T1D), type 2 diabetes (T2D), and gestational diabetes (GD).3 In all three types, the use of insulin therapy to help regulate BG is critical. In T1D, this therapy supplies the body with the insulin it is unable to produce. In T2D and GD, insulin may be used later in the therapeutic process to stabilize the concentration of BG.3 One significant advance in the pharmacologic treatment of diabetes is the approval of biosimilars for insulin glargine (Lantus), a long-acting type of insulin. The first biosimilar insulin, Semglee (insulin glargine-yfgn), was approved in July 2021; this was followed by approval of the second biosimilar, Rezvoglar (insulin glargine-aglr), in December 2021.4
Diabetes Overview
The pathophysiology of diabetes centers around insulin’s role in the body. Insulin, which is made by the pancreas, is an essential hormone that enables glucose to enter muscle and adipose cells for conversion to energy; it also stimulates glycogen and fatty-acid storage and has other metabolic effects.5 Normally, rises in BG stimulate the pancreatic beta cells to release insulin, with excess glucose stored in the liver as a form of glycogen.6 However, depending on the type of diabetes, either an insulin deficiency exists or the peripheral tissues are resistant to the insulin.5
GD is a common pregnancy complication that usually resolves after childbirth.7 In GD, hormonal and other metabolic changes that occur during pregnancy affect the cells’ response to insulin.7 Obesity and overweight have been linked to GD.7
In T1D, the pancreas loses the capacity to produce—or produces very little—insulin.8 This form of diabetes is an autoimmune disease wherein the patient’s immune system misidentifies and attacks the pancreatic beta cells, destroying them.8 T1D is most commonly diagnosed in childhood, but it can develop in adulthood as well.9 Currently no cure exists for T1D, and therapy focuses on maintaining sufficient BG and preventing complications. Lifelong insulin replacement is the primary treatment for these patients.8
The most common form of diabetes is T2D. The cells start responding poorly to the released insulin, leading to insulin resistance; in turn, the pancreas produces more insulin in an attempt to trigger a cellular response.10 This malfunctioning feedback loop results in abnormally high BG.10 One distinction between T1D and T2D is the patient population most commonly affected; middle-aged and older persons are more likely than young people to develop T2D.7 Risk factors for T2D are classified as nonmodifiable or modifiable. Ethnicity and genetic predisposition are nonmodifiable risk factors, whereas obesity, unhealthy diet, and lack of physical activity are modifiable risk factors.10
The primary goal of T2D management is glycemic control. This can be determined by measuring the patient’s A1C (i.e., glycated hemoglobin) and BG. An A1C test measures the average BG concentration over a 3-month period.11 According to the American Diabetes Association, the recommended A1C is <7%; the BG goal range is 70 mg/dL to 180 mg/dL for >70% of the time, with a level <70 mg/dL for <4% of the time.11
Pharmacologic Therapy
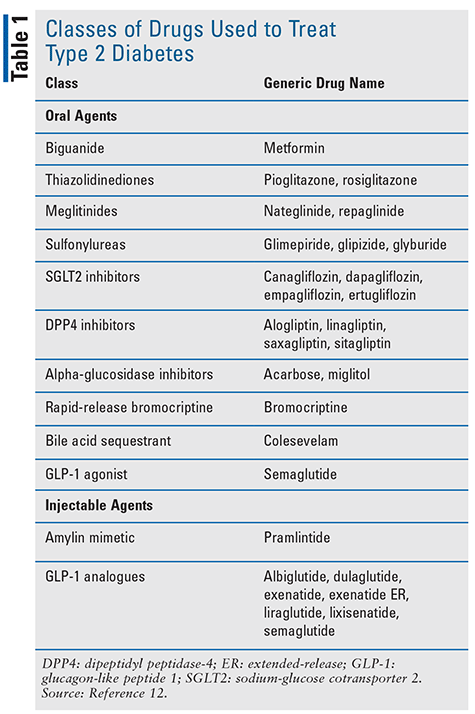
In addition to lifestyle modifications involving exercise and diet, an array of pharmacologic agents are available. TABLE 1 lists the classes of pharmacologic agents available for the treatment of T2D. Drugs from each class may be used as monotherapy, or combination therapy may be tried if monotherapy did not effect any significant changes.12
Insulin Therapy and Biosimilars
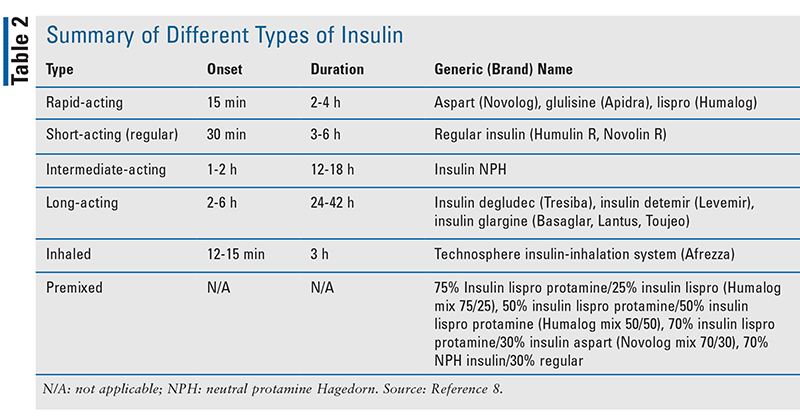
When insulin was discovered in 1921, it became the first diabetes treatment.13 Since then, insulin therapy has been critically important in managing BG and preventing complications in both T1D and T2D. In T1D, this therapy provides the insulin the body cannot produce. In T2D patients, insulin therapy may be initiated after a trial of other available options or used in a combination regimen.11 TABLE 2 summarizes the different types of insulin and selected pharmacokinetic properties.8
A biosimilar is a biological product that does not differ clinically from the existing FDA-approved product.14 An abbreviated licensure pathway for biological products showing biosimilarity or interchangeability was approved under Section 351(k)(7)(C) of the Public Health Service Act.15 The introduction of insulin biosimilars can potentially lower costs and increase medication access for diabetic patients.16 The following sections discuss insulin glargine (Lantus) and the two recently approved insulin glargine biosimilars, Semglee and Rezvoglar.
Lantus
Lantus (insulin glargine) is a subcutaneous long-acting insulin analogue that is indicated for the improvement of glycemic control in patients with T1D or T2D. This agent should not be used to treat diabetic ketoacidosis, a serious complication involving the production of ketones.17 Insulin glargine should be employed in conjunction with a short-acting insulin in T1D patients. The typical dose is one-third of the total daily insulin requirement. The starting dose for T2D patients is 0.2 units/kg, or 10 units once daily.17 Lantus is available in 10-mL multiple-dose vials and 3-mL single-use SoloStar prefilled pens with a concentration of 100 units/mL.17 Potential adverse events (AEs), such as hypoglycemia and hypokalemia, should be discussed with patients.17
Semglee
Semglee (insulin glargine-yfgn), the first interchangeable biosimilar insulin product, was approved by the FDA in July 2021.18 This product is interchangeable with Lantus (insulin glargine), its reference product. This means that Semglee may be used in place of Lantus without obtaining the prescriber’s approval.14 Like insulin glargine, insulin glargine-yfgn is a long-acting insulin analogue, and it is administered subcutaneously once daily. Insulin glargine-yfgn should not be diluted or mixed with other types of insulin or solution. The product is available in two formulations: 10-mL vials and 3-mL prefilled disposable pens.18
Proving a product’s interchangeability requires additional product testing.14 Approval of Semglee was based on clinical studies that demonstrated the product’s similarity to the reference product in terms of purity, efficacy, and safety.18 Clinical evidence also indicates that this biosimilar will exhibit the same clinical results in patients.18 When Semglee was approved, Acting FDA Commissioner Janet Woodcock, MD, stated, “Today’s approval of the first interchangeable biosimilar product furthers FDA’s longstanding commitment to support a competitive marketplace for biological products and ultimately empowers patients by helping to increase access to safe, effective and high-quality medications at potentially lower cost.”18
INSTRIDE 1 Study: INSTRIDE 1 was the first clinical trial of insulin glargine-yfgn and its properties. This 52-week open-label, randomized, phase III study assessed the safety and efficacy of insulin glargine-yfgn (also known as MYL-1501D) as a potential insulin glargine biosimilar in T1D patients.19 The 558 participants were randomly assigned to receive either insulin glargine (Lantus) or MYL-1501D in addition to mealtime insulin lispro, and treatment outcomes were determined by A1C. The primary objective was to ascertain whether MYL-1501D was noninferior to insulin glargine at the end of 24 weeks. The secondary end points were changes in fasting BG, insulin doses, self-monitored BG, and potential AEs up to week 52. For the primary objective, the average A1C for MYL-1501D was 0.14% (standard error [SE]: 0.054; 95% CI 0.033, 0.244), and for the reference product it was 0.11% (SE: 0.054; 95% CI 0.007, 0.220).19 Based on the similar values, MYL-1501D had comparable safety and efficacy outcomes to the reference product. There were no clinically significant between-group differences in secondary end points, including AEs such as hypoglycemia. Overall, MYL-1501D was well tolerated among these T1D patients throughout the 52-week study period.19
INSTRIDE 2 Study: INSTRIDE 2, the second pivotal trial of insulin glargine-yfgn, compared the safety and efficacy of MYL-1501D with insulin glargine (Lantus) in 560 insulin-naïve and nonnaïve T2D patients.20 In this open-label, multicenter, randomized, parallel-group, phase III noninferiority study, the insulin was administered in addition to oral antidiabetic medications for 24 weeks. The primary objective was to assess efficacy by monitoring the changes in A1C up to week 24. The secondary end points, as in INSTRIDE 1, were to evaluate changes in fasting plasma glucose, self-monitored BG, AEs, and other variables. From baseline to 24 weeks, the average A1C was –0.60% (95% CI –0.78, –0.41) and –0.66% (95% CI: –0.84, –0.48) for MYL-1501D and the reference product, respectively. MYL-1501D was determined to be noninferior to the reference product based on the results and clinical outcomes.20
Rezvoglar
Rezvoglar (insulin glargine-aglr) was approved in December 2021 as the second insulin glargine biosimilar. This long-acting basal insulin is indicated for adults and children with T1D as well as for adults with T2D.21 Unlike Semglee, Rezvoglar requires the prescriber’s approval before dispensing; this is because insulin glargine-yfgn is an interchangeable biosimilar and insulin glargine-aglr is not.4 The readily available formulation is a 3-mL prefilled KwikPen (100 mg/mL), with once-daily subcutaneous administration.21 This product, like insulin glargine-yfgn, should not be diluted or mixed with other types of insulin or solutions.21
Because it is a biosimilar, insulin glargine-aglr has a mechanism of action that is very similar to that of insulin glargine. As previously noted, insulin’s primary function is to regulate glucose metabolism. The stimulation of peripheral glucose uptake and inhibition of hepatic glucose production reduce the BG concentration. The use of insulin glargine-aglr to treat diabetic ketoacidosis is not advised.21
As with all insulin products, hypoglycemia is the most common AE. Insulin glargine-aglr can cause or worsen heart failure, and the use of thiazolidinediones (an oral class of antidiabetic agents) in combination with Rezvoglar will increase the risk.21 Clinical trials are currently being conducted to assess insulin glargine-aglr’s safety and efficacy.
Future Developments
Semglee and Rezvoglar may be joined by an additional insulin biosimilar in the future. In 2021, Lannett Company submitted an Investigational New Drug application with the FDA for a pivotal clinical trial of a potential biosimilar insulin glargine product currently in development.4 The company hopes to file a Biologics Licensing Application in 2023, with a potential launch estimated for 2024.4
Conclusion
Insulin is a staple medication used by diabetic patients for BG control. A comprehensive understanding of insulin biosimilars and interchangeable biosimilar products enables healthcare professionals to offer patients insulin options that are both efficacious and affordable. Semglee, the first interchangeable insulin biosimilar, offers a lower-cost alternative to insulin glargine. The subsequent introduction of the insulin biosimilar Rezvoglar gives diabetic patients an additional choice of therapeutic agents to help them achieve better glycemic control.
REFERENCES
1. CDC. National Diabetes Statistics Report. www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed May 12, 2022.
2. National Institute of Diabetes and Digestive and Kidney Diseases. What is diabetes? www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes. Accessed April 1, 2022.
3. Mayo Clinic. Diabetes treatment: using insulin to manage blood sugar. www.mayoclinic.org/diseases-conditions/diabetes/in-depth/diabetes-treatment/art-20044084. Accessed April 1, 2022.
4. Center for Biosimilars. Rezvoglar broadens insulin glargine biosimilar options. www.centerforbiosimilars.com/view/rezvoglar-broadens-insulin-glargine-biosimilar-options. Accessed March 1, 2022.
5. Moini J. Epidemiology of Diabetes. New York, NY: Elsevier Science; 2019:25-43.
6. Wexler DJ. Patient education: type 2 diabetes: insulin treatment (beyond the basics). UpToDate. www.uptodate.com/contents/type-2-diabetes-insulin-treatment-beyond-the-basics. Accessed April 1, 2022.
7. McIntyre HD, Catalano P, Zhang C, et al. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5(1):47.
8. Lucier J, Weinstock RS. Diabetes mellitus type 1. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. www.ncbi.nlm.nih.gov/books/NBK507713. Accessed March 17, 2022.
9. CDC. Type 1 diabetes. www.cdc.gov/diabetes/basics/type1.html. Accessed March 17, 2022.
10. Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):6275.
11. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S83-S96.
12. Feingold KR. Oral and injectable (non-insulin) pharmacological agents for the treatment of type 2 diabetes. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc; 2022.
13. Vecchio I, Tornali C, Bragazzi NL, Martini M. The discovery of insulin: an important milestone in the history of medicine. Front Endocrinol (Lausanne). 2018;9:613.
14. FDA. Biosimilar and interchangeable products. www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products#biological. Accessed March 14, 2022.
15. FDA. Reference product exclusivity for biological products filed under Section 351(a) of the PHS Act. www.fda.gov/regulatory-information/search-fda-guidance-documents/reference-product-exclusivity-biological-products-filed-under-section-351a-phs-act. Accessed March 15, 2022.
16. Dolinar RO, Edelman S, Heinemann L, et al. Impact of biosimilar insulins on clinical practice: meeting report. J Diabetes Sci Technol. 2014;8(1):179-185.
17. Lantus (insulin glargine) package insert. Bridgewater, NJ: sanofi-aventis U.S. LLC; January 2021.
18. FDA. FDA approves first interchangeable biosimilar insulin product for treatment of diabetes. www.fda.gov/news-events/press-announcements/fda-approves-first-interchangeable-biosimilar-insulin-product-treatment-diabetes. Accessed March 1, 2022.
19. Blevins TC, Barve A, Sun B, Ankersen M. Efficacy and safety of MYL-1501D vs insulin glargine in patients with type 1 diabetes after 52 weeks: results of the INSTRIDE 1 phase III study. Diabetes Obes Metab. 2018;20(8):1944-1950.
20. Blevins TC, Barve A, Sun B, et al. Efficacy and safety of MYL-1501D versus insulin glargine in patients with type 2 diabetes after 24 weeks: results of the phase III INSTRIDE 2 study. Diabetes Obes Metab. 2019;21(1):129-135.
21. Rezvoglar (insulin glargine-aglr) package insert. Indianapolis, IN: Eli Lilly & Co; December 2021.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






