ABSTRACT: Sodium-glucose cotransporter 2 (SGLT2) inhibitors target the proximal tubule where the SGLT2 receptor is located. By blocking the receptor, glucose is forced out of the body through the urine and can no longer reabsorb into the body. In patients with atherosclerotic cardiovascular disease or heart failure, SGLT2 inhibitors have been seen to be effective in decreasing overall morbidity and mortality. Although the direct mechanism for how SGLT2 inhibitors work to help cardiovascular diseases is unknown, the benefits are shown in the data. SGLT2 inhibitors were originally approved for use in patients with type 2 diabetes; however, some are now approved to reduce the risk of end-stage kidney disease, cardiovascular death, and hospitalization. Most common side effects experienced include vulvovaginal candidiasis in women and urinary tract infections.
Over the past few years, there has been an increase in the use of sodium-glucose cotransporter 2 (SGLT2) inhibitors in patients with and without diabetes. SGLT2 inhibitors were originally approved for glycemic control in patients with diabetes.1 The current SGLT2 inhibitors on the market are displayed in TABLE 1, and the benefits of therapy are summarized in TABLE 2.2-6 The mechanism by which SGLT2 inhibitors work is by blocking the SGLT2 receptor in the proximal tubule to prevent reabsorption of glucose back into the body. The glucose is therefore excreted in the urine.7 Due to glucose being small enough to pass through the glomerulus, it will normally be excreted through the urine if it is not reabsorbed through the cells in the tubule wall. This system begins to break down in individuals who have elevated blood glucose levels, resulting in glucosuria.
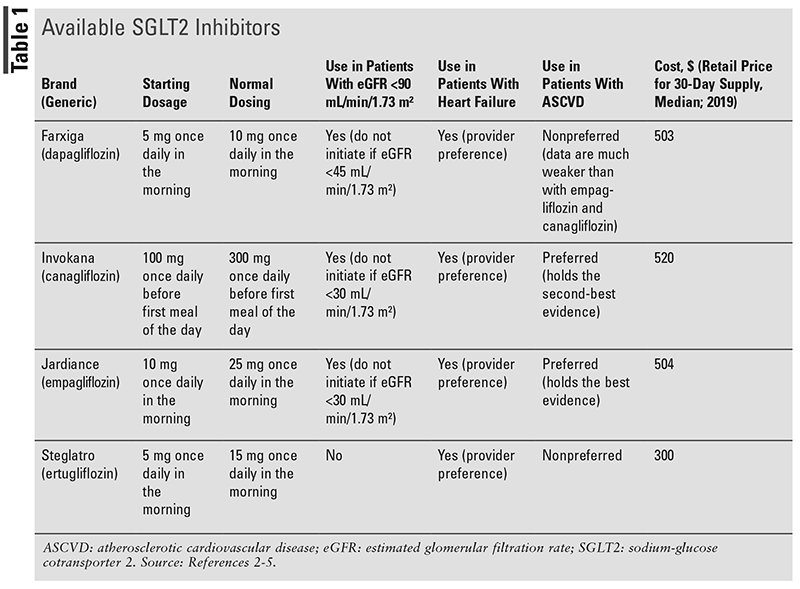
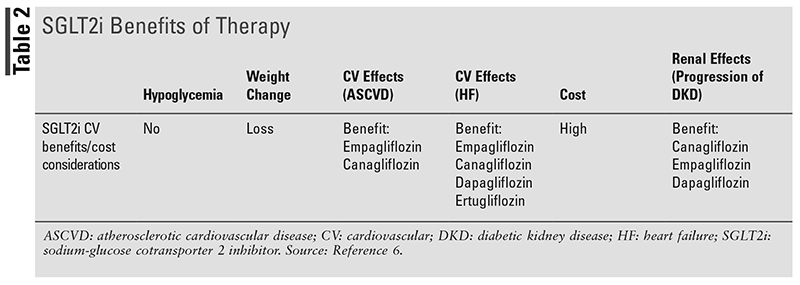
The details of how the kidney cells reabsorb glucose were discovered in the 1980s through the identification of differences in glucose transport in rat kidney tubules. Researchers identified the gene for the intestinal glucose transport protein that belonged to SGLTs. The intestinal protein was named SGLT1, and after discovering a second, closely related protein, it was named SGLT2. SGLT2 is located early in the tubule and is a high-capacity glucose transporter (90%) compared with SGLT1, which is found later in the tubule and is a low-capacity transporter (10%).7 Studies over the past few years have started to look at them in the context of other disease states, such as chronic kidney disease (CKD), heart failure (HF), and atherosclerotic cardiovascular disease (ASCVD). Recent results looking at both cardiovascular benefits and renal benefits have shown positive outcomes for SGLT2 inhibitors. There are four medications currently on the market as brand-name products: dapagliflozin (Farxiga), canagliflozin (Invokana), empagliflozin (Jardiance), and ertugliflozin (Steglatro). The first SGLT2 inhibitor approved by the FDA was Invokana in March 2013.7 Recent studies have shown possible mechanisms for cardiovascular benefit and include early natriuresis leading to reduced plasma volume, increased hematocrit, and lower blood pressure, as well as improved overall vascular function.
Side effects that have occurred due to the use of SGLT2 inhibitors include vulvovaginal candidiasis in 10% to 15% of women, frequent urinary tract infections (UTIs) in around 8% of all patients, lower limb amputations in about 2.7% of patients, and increased risk of fracture in 1.5% of patients taking canagliflozin.8-11 Monitoring parameters include renal function (baseline and periodically throughout), blood pressure, volume status (electrolytes, hematocrit), glycemic indices (A1C, blood glucose), and signs and symptoms of diabetic ketoacidosis (nausea, vomiting, malaise).
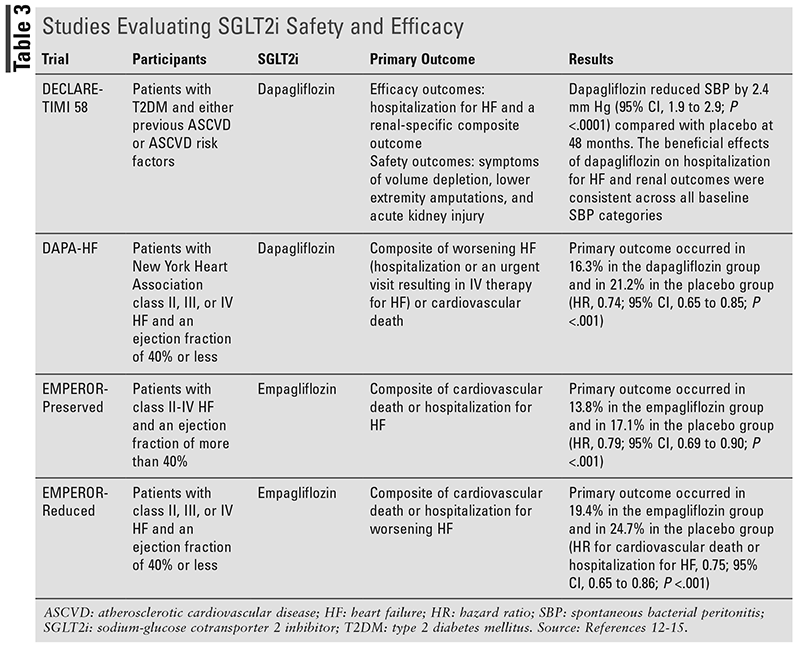
A significant amount of research has been performed to evaluate the safety and efficacy of SGLT2 inhibitors. More recent evidence that supports the use of SGLT2 inhibitors in those with and without diabetes includes the DECLARE-TIMI 58, DAPA-HF, EMPEROR-Preserved, and EMPEROR-Reduced studies.12-14
DECLARE-TIMI 58
The 2021 DECLARE-TIMI 58 trial looked at cardiovascular, renal, and metabolic benefits of dapagliflozin versus placebo.12 It was broken down into different cohorts, including the primary cardiovascular prevention cohort looking at prospective benefits of patients with ASCVD or primary prevention in high-risk patients with multiple risk factors (MRF). The primary outcome looked for worsening of events, including cardiovascular death/hypertensive HF (CVD/HHF), as well as major adverse cardiovascular events (which included CVD, myocardial infarction, and ischemic stroke). In patients with MRF treated with dapagliflozin, there was a reduction in the risk of CVD/HHF, and this did not differ from patients with ASCVD. This means that there was an increase in proactive cardiovascular behavior among patients taking dapagliflozin versus patients not taking dapagliflozin.
DAPA-HF
DAPA-HF was a phase III, placebo-controlled trial in which 4,744 patients with New York Heart Association class II, III, or IV HF and an ejection fraction of £40% were randomly assigned to receive dapagliflozin 10 mg daily (2,372 patients) or placebo (2,371 patients).13 The primary outcome was a composite of worsening HF or death from cardiovascular causes, which occurred in 16.3% of patients in the dapagliflozin group and 21.2% in the placebo group (P <.001). Secondary outcomes included hospitalization for HF or cardiovascular death, a composite of worsening renal function (sustained decline in eGFR of ³50%, end-stage renal disease , or renal death), and death from any cause. Hospitalization for HF was 9.7% of the dapagliflozin group compared with 13.4% in the placebo group. Death from cardiovascular causes occurred in 9.6% of dapagliflozin patients and 11.5% of placebo patients. A total of 11.6% of patients in the dapagliflozin group and 13.9% in the placebo group died from any cause (hazard ratio [HR] 0.83; 95% CI, 0.71-0.97). In summary, patients taking dapagliflozin had better results in each outcome category.
EMPEROR-Preserved
The EMPEROR-Preserved trial was a double-blind study that randomly assigned 5,988 patients with class II-IV HF and an ejection fraction of more than 40% to receive empagliflozin 10 mg once daily or placebo.14 This study was performed to evaluate the effects of empagliflozin inhibiting SGLT2 on HF outcomes in patients with HF with a preserved ejection fraction (HFpEF). The primary outcome was a composite of adjudicated cardiovascular death or hospitalization for HF. The primary outcome occurred in 13.8% of the empagliflozin group and in 17.1% in the placebo group (P <.001). Hospitalization for HF occurred in 8.6% of the empagliflozin group and 11.8% of the placebo group. Death from cardiovascular causes occurred in 7.3% in the empagliflozin group and in 8.2% in the placebo group. Hospitalizations for HF were lower in the empagliflozin group compared with the placebo group (P <.001). SGLT2 inhibition with empagliflozin decreased relative risk in the composite of cardiovascular death or hospitalization for HF by 21%.
EMPEROR-Reduced
The EMPEROR-Reduced trial was a randomized, double-blind, placebo-controlled trial that evaluated patients over age 18 years who had chronic HF (functional class II, III, or IV) with a left ventricular ejection fraction of £40%. The primary outcome was a composite of adjudicated CVD or hospitalization for HF. The primary outcome occurred in 19.4% of empagliflozin patients and in 24.7% of placebo patients (P <.001). The effect of empagliflozin on the primary outcome was consistent among all subgroups, including patients with and without diabetes at baseline. The risk of CVD was reduced by 8% in the empagliflozin group compared with placebo.15
The above studies, shown in TABLE 3, show the use of SGLT2 inhibitors is associated with a reduction in the risk of CVD/HHF, the likelihood of worsening HF, death from cardiovascular causes, and hospitalization.12-15 Therefore, the use of SGLT2 inhibitors should be recommended in accordance with the guidelines.
Guidelines
The joint recommendations cite DAPA-HF and EMPEROR-HF as evidence of the drug class’ benefit for differing stages of HF. Moreover, according to the 2022 AHA/ACC/HFSA guidelines, the recommended guideline-directed medical therapy for patients with HF with reduced ejection fraction (HFrEF) is the use of SGLT2 inhibitors. The newest recommendation is that in patients with symptomatic chronic HFrEF, an SGLT2 inhibitor is a 1A recommendation to decrease hospitalizations and cardiovascular mortality. This guideline specifically references the DAPA-HF and EMPEROR-Reduced trials, which show the benefit of using dapagliflozin and empagliflozin in HF patients with or without type 2 diabetes. Patients with an estimated glomerular filtration rate <20 mL/min/1.73 m2 for empagliflozin and <30 mL/min/1.73 m2 for dapagliflozin are not recommended to receive this therapy. The benefit of using these novel therapies in HFrEF is the minimal side effects, intermediate economic value, and its prevention of overall mortality.9 The FDA approved dapagliflozin for use in adult patients with HFrEF in May 2017. The FDA also approved empagliflozin for use in adults with HFrEF in August 2021. More recently, in February 2022, the FDA approved empagliflozin for the use in HFpEF. The approval was indicated to decrease CVD risk and hospitalizations, similar to that in HFrEF. This decision was based on data from the EMPEROR-Preserved trial.16 As expressed above, the DECLARE-TIMI 58 trial looked more at dapagliflozin use in patients with ASCVD. Although there has not been a specific approval for ASCVD, data from the DECLARE-TIMI 58 showed a reduction in risk of MACE in patients with cardiovascular disease. The reduced risk for cardiovascular disease was directly driven by decrease in HHF, adding to data that support SGLT2 inhibitor use in HF patients to decrease risk of cardiovascular-related mortality.17-19
Based on findings from various trials that have been conducted on SGLT2 inhibitors, it is clear that their use results in cardiovascular benefits. Although the mechanism by which cardiovascular benefits are indicated is not clear, there is an increasing amount of literature leading towards a better understanding of the relationship.
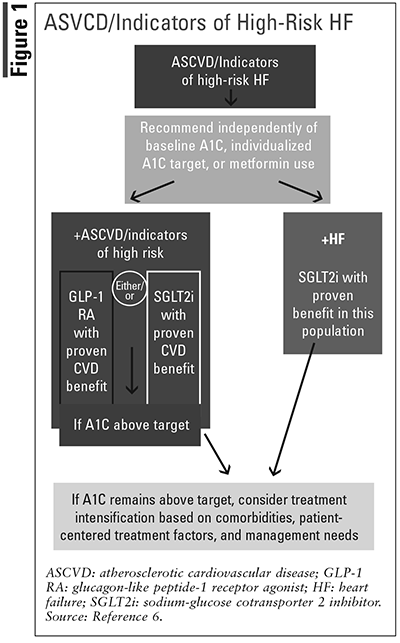
As pharmacists, it is important to stay up to date with new drugs and indications. Selection of therapy based on patient factors can be guided by TABLE 3 and FIGURE 1.6 As SGLT2 inhibitors become a more popular choice in patients with diabetes, it is important to understand the alternative benefits to these drugs so they can be communicated to the patient. Many patients with type 2 diabetes also have ASCVD or HF. It can be helpful to lessen patient pill burden by targeting multiple disease states with one drug. Also, with once-daily dosing and minimal side effects, an SGLT2 inhibitor becomes a possible evidence-based option. The added cardiovascualr protective benefit that comes with SGLT2 inhibitors can have a large impact on decreasing morbidity and mortality over time in this patient population. It is important to explain to patients how SGLT2 inhibitors can act as a protective agent for several organs, including the heart and kidneys.

Counseling on potential side effects is important when patients are started on these medications. Side effects of SGLT2 inhibitors include frequent UTIs, vulvovaginal candidiasis, and acute kidney injuries. Although they are not curative for patients with these disease states, SGLT2 inhibitors are another option to manage these chronic conditions. At this time, these drugs are available brand only and can be expensive for many patients. However, the benefit from using these drugs may outweigh the extreme cost in many situations, and it is important to be able to correctly inform patients of the positive outcomes associated with SGLT2 inhibitors.
Conclusion
Overall, SGLT2 inhibitors are becoming more commonly utilized for treating patients with cardiovascular disease with or without diabetes. Current trial data show that SGLT2 inhibitors can decrease both morbidity and mortality in patients with HF and ASCVD. The minimal occurrence of side effects, once-a-day dosing, and oral formulations make them a popular choice with patients as well. Although the exact mechanisms for how SGLT2 inhibitors can be beneficial to patients with cardiovascular diseases is unknown, their benefits still outweigh any possible risks in the general population.
REFERENCES
1. Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020;17(12):761-772.
2. UpToDate. Canagliflozin: drug information. ASA Monitor. 2021. www.uptodate.com/contents/canagliflozindrug-information?search=canagliflozin&source=panel_search_result&selectedTitle=1~36&usage_type=panel&kp_tab=drug_general&display_rank=1.
3. UpToDate. Dapagliflozin: drug information. ASA Monitor. 2021. www.uptodate.com/contents/dapagliflozindrug-information?search=dapagliflozin&topicRef=93590&source=related_link.
4. UpToDate. Empagliflozin: drug information. ASA Monitor. 2021;85(10):23-23. www.uptodate.com/contents/empagliflozin-drug-information?source=auto_suggest&selectedTitle=1~4---1~4---empa&search=empagliflozin.
5. UpToDate. Ertugliflozin: drug information. ASA Monitor. 2021;85(10):23-23. www.uptodate.com/contents/ertugliflozin-drug-information?source=auto_suggest&selectedTitle=2~4---2~4---ert&search=ertugliflozin.
6. American Diabetes Association Professional Practice Committee. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2021;45(suppl 1):S125-S143.
7. National Institute of Diabetes and Digestive and Kidney Diseases. Story of discovery: SGLT2 inhibitors: harnessing the kidneys to help treat diabetes. www.niddk.nih.gov/news/archive/2016/story-discovery-sglt2-inhibitors-harnessing-kidneys-help-treat-diabetes. Accessed September 2, 2022.
8. Hollander P, Liu J, Hill J, et al. Ertugliflozin compared with glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin: the VERTIS SU randomized study. Diabetes Ther. 2018;9(1):193-207.
9. Clar C, Gill JA, Court R, Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open. 2012;2(5):e001007.
10. Watts NB, Bilezikian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101(1):157-166.
11. Ueda P, Svanström H, Melbye M, et al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ. 2018;363:k4365.
12. Furtado RHM, Raz I, Goodrich EL, et al. Efficacy and safety of dapagliflozin in type 2 diabetes according to baseline blood pressure: observations from DECLARE-TIMI 58 trial. Circulation. 2022;145(21):1581-1591.
13. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008.
14. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461.
15. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424.
16. FDA. FDA approves treatment for wider range of patients with heart failure. February 24, 2022. www.fda.gov/news-events/press-announcements/fda-approves-treatment-wider-range-patients-heart-failure. Accessed October 24, 2022.
17. Luo J, Feldman R, Rothenberger SD, et al. Coverage, formulary restrictions, and out-of-pocket costs for sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists in the Medicare Part D program. JAMA Netw Open. 2020;3(10):e2020969.
18. Campbell P. FDA approves empagliflozin for treatment in adults with heart failure. Practical Cardiology. February 24, 2022. www.practicalcardiology.com/view/fda-approves-empagliflozin-for-treatment-in-adults-with-heart-failure. Accessed October 24, 2022.
19. Melillo G. FDA approves dapagliflozin to treat heart failure, breaking new ground in SGLT2 competition. AJMC. May 6, 2020. www.ajmc.com/view/fda-approves-dapagliflozin-to-treat-heart-failure-breaking-new-ground-in-sglt2-competition. Accessed October 24, 2022.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





