US Pharm. 2019;44(7):26-29.
ABSTRACT: Influenza is a common viral infection that occurs largely in the winter months. The Infectious Diseases Society of America recently released updated clinical practice guidelines for the management of seasonal influenza. Recommended antivirals for uncomplicated influenza in otherwise healthy patients in the outpatient setting include oral oseltamivir, inhaled zanamivir, and IV peramivir. Oseltamivir is preferred for hospitalized patients. Following publication of the guidelines, baloxavir marboxil was approved for outpatient treatment of uncomplicated influenza in persons aged 12 years and older. For prevention of influenza, recommended antivirals include oseltamivir and zanamivir. Pharmacists are in a key position to ensure that patients receive an annual influenza vaccine and to recommend appropriate antiviral therapy for the treatment and prophylaxis of influenza.
Two kinds of influenza virus—types A and B—cause most cases of flu, a respiratory illness that remains a significant cause of morbidity and mortality.1-3 In the United States, the influenza season (the period when influenza activity usually occurs) starts in October, peaks between December and February, and lasts through May.1,4 The CDC estimates that between October 1, 2018, and April 13, 2019, there were 16.7 million to 19.4 million medical visits for influenza, 500,000 to 600,000 hospitalizations, and 34,000 to 58,000 influenza-related deaths in the U.S.2 Recently, the Infectious Diseases Society of America (IDSA) released updated clinical practice guidelines for the management of seasonal influenza.
Clinical Presentation and Complications
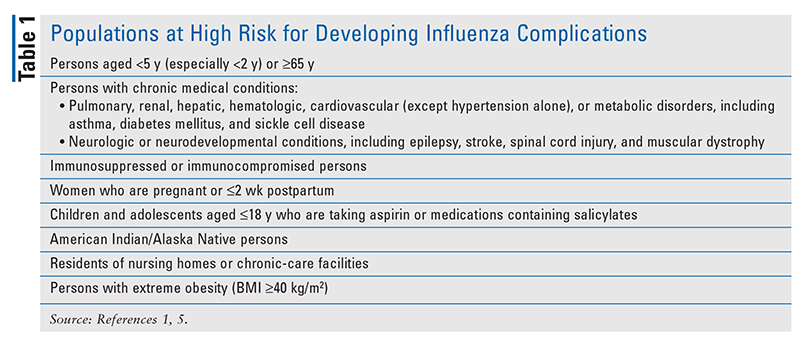
Patients with uncomplicated influenza typically present with an abrupt onset of symptoms, including fever (although this is not always present), cough, chills, muscle aches, fatigue or weakness, chest pain, headache, and nasal congestion.1,5 Infants and children may present with the additional symptom of diarrhea.1,5 It is important to note that elderly persons, infants, and immunocompromised patients may have an atypical presentation (e.g., unexplained fever or exacerbation of a chronic condition such as asthma or heart failure) or present with a complication of influenza (e.g., pneumonia).1 Although most people completely recover from influenza, the patient populations listed in TABLE 1 may experience complications ranging from upper respiratory tract infections such as sinusitis and otitis media to pneumonia, respiratory failure, and sepsis.1,5
Diagnosis
The diagnosis of influenza virus may be made based on the patient’s presenting symptoms and the healthcare provider’s clinical judgment, or the provider may choose to use a diagnostic influenza test.6 A patient with clinical symptoms of influenza may not be tested if the results would not influence clinical management, including decisions on the use of antibiotic or antiviral medication, the need for further diagnostic testing, and recommendations for household contacts.6 The latest guidelines on seasonal influenza include recommendations for influenza testing in both outpatient and inpatient settings. During periods of influenza activity, clinicians should test for influenza in the outpatient or emergency department setting in patients who are at high risk for developing complications or who present with acute onset of respiratory symptoms and either exacerbation of a chronic medical condition or a known complication of influenza (e.g., pneumonia) if the testing result will influence clinical management. Clinicians may consider testing in patients not at high risk for complications who present with respiratory symptoms or symptoms consistent with influenza if the results will influence decisions related to treatment or chemoprophylaxis of high-risk household contacts. It is recommended that the patient be tested as soon as possible after illness onset because a decrease in viral shedding occurs over time, which may result in a false-negative result if the test is performed more than 4 days after symptom onset.1
During periods of influenza activity, clinicians should test for influenza upon admission in all patients requiring hospitalization for acute respiratory illness (including pneumonia); those with acute worsening of chronic cardiopulmonary disease; those who are immunocompromised or considered at high risk for influenza complications who present with acute onset of nonspecific respiratory symptoms or unexplained fever; and those who develop acute onset of respiratory symptoms while hospitalized. During periods of low influenza activity, testing should be performed upon admission in all patients hospitalized for acute respiratory illness who have an epidemiological link to an individual or location with known influenza.1
Several tests are available for diagnosing influenza virus. Rapid influenza diagnostic tests (RIDTs) work by detecting viral antigens, and results are available within 15 minutes; however, these tests have variable sensitivity and may give a negative result even if influenza is present. Therefore, the latest guidelines recommend the use of rapid molecular assays (such as nucleic acid amplification tests) over RIDTs in the outpatient setting. Molecular assays detect influenza viral RNA, provide results within 15 to 30 minutes, and have high sensitivity and specificity, with a greater ability to detect influenza virus. For hospitalized patients, molecular assays are recommended, including multiplex reverse transcription polymerase chain reaction assays that target a panel of respiratory pathogens, including influenza virus. RIDTs should not be used in hospitalized patients when more sensitive molecular assays are available; when they are used, however, follow-up testing should be performed to confirm negative results.1
Treatment
Early antiviral treatment can shorten the duration of symptoms or hospitalization and reduce the risk of influenza-related complications.7 Therefore, antiviral medications should be initiated as early as possible (ideally within 48 hours of symptom onset). Additionally, because antivirals may benefit patients with severe or progressive illness and hospitalized patients even when initiated more than 48 hours after illness onset, treatment is recommended in these patients regardless of duration of illness. A patient’s vaccination status should not affect the decision to start antiviral therapy, and treatment should be initiated before laboratory confirmation of influenza.1,7
For those with suspected or documented influenza, the latest IDSA guidelines recommend that the following populations be treated with antivirals as soon as possible regardless of influenza-vaccination history: patients of any age who require hospitalization for influenza (regardless of duration of illness prior to hospitalization); outpatients of any age with severe or progressive illness (regardless of duration of illness); outpatients at high risk for complications; children younger than 2 years old; adults aged 65 years and older; pregnant women; and women who are 2 weeks or less postpartum. Antiviral treatments may be considered in previously healthy outpatients (i.e., not at risk for complications) whose illness started 2 days or less before presentation; symptomatic outpatients with household contacts considered to be at high risk for complications; and symptomatic healthcare providers who care for high-risk patients.1
For uncomplicated influenza in otherwise healthy outpatients, the updated guidelines recommend a single neuraminidase inhibitor (oral oseltamivir, inhaled zanamivir, or IV peramivir). These agents work by preventing the release of newly formed viral particles from infected cells.8,9 For hospitalized patients, the guidelines recommend oral or enterically administered (via nasogastric or orogastric tube) oseltamivir.1,7 TABLE 2 summarizes available antiviral agents for the treatment and prevention of influenza virus.1,7,8,10
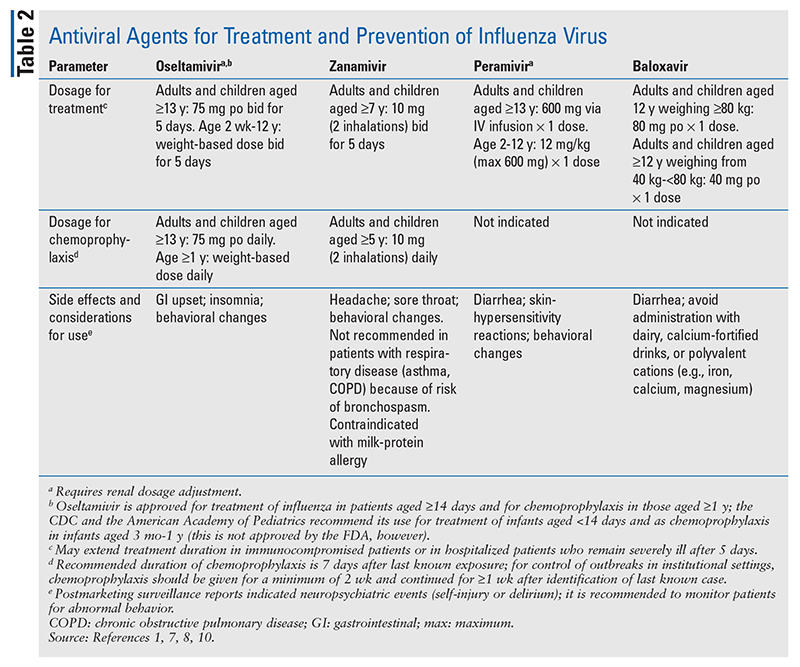
Baloxavir marboxil is an anti-influenza agent with a long half-life that was approved in October 2018 (after the guidelines were finalized) as a single oral dose for the treatment of uncomplicated influenza in patients aged 12 years and older who have had symptoms for less than 2 days.1,11 Baloxavir marboxil is an oral prodrug of baloxavir, which is an endonuclease inhibitor that inhibits the activity of a polymerase acidic protein that is required for viral gene transcription, thus inhibiting replication of the influenza virus.11 Clinical trials of baloxavir marboxil included outpatients aged 12 to 64 years and excluded patients with underlying medical conditions, those aged 65 years and older, and those who required hospitalization for influenza.7,9,12 At this time, it is not known whether baloxavir marboxil is safe in women who are pregnant or breastfeeding, and there are no data on its use for the treatment of patients hospitalized with influenza.7,9,12
Patients infected with influenza virus may also have a bacterial coinfection. The updated guidelines state that clinicians should evaluate for, and empirically treat, bacterial coinfection in patients with severe disease and those who clinically worsen after initial improvement with antiviral therapy. Additionally, bacterial coinfection should be considered in patients without clinical improvement after 3 to 5 days of antiviral therapy.1
Prevention
Annual receipt of an influenza vaccine in all individuals aged 6 months and older who do not have any contraindications is the best way to help prevent influenza infection.3,7 Prophylaxis with antiviral medications in the outpatient setting should not be used as a substitute for vaccination, but it may be considered for influenza prevention in adults and children aged 3 months and older in selected situations.1,3,7 The recommended antiviral agents for chemoprophylaxis are oral oseltamivir and inhaled zanamivir (TABLE 2).
Patients in whom preexposure antiviral chemoprophylaxis may be considered for the duration of the influenza season include those at high risk for influenza complications who have a contraindication to the influenza vaccine; those who may have an inadequate antibody response to the vaccine (e.g., are significantly immunocompromised); and those who are at highest risk for complications, including lung-transplant recipients and those within 6 to 12 months of receiving a hematopoietic stem cell transplant. Short-term antiviral chemoprophylaxis may be considered in patients who have not yet achieved immunity via vaccination (it takes approximately 2 weeks after vaccination to develop a protective antibody response) and in unvaccinated individuals who are household contacts of someone who is unable to receive chemoprophylaxis and is at very high risk for influenza-related complications.1,7
Postexposure antiviral chemoprophylaxis may be considered in the outpatient setting for adults and children aged 3 months and older after exposure to influenza (especially those who are at high risk for influenza-related complications and have not received the vaccine, or those in whom the vaccine is contraindicated or expected to be less effective). If antiviral chemoprophylaxis is used, it should be started as soon as possible and no later than 48 hours after exposure to influenza, and patients should be counseled to immediately seek medical attention if they develop a febrile respiratory illness so that they can be evaluated for influenza infection.1,7
In institutional settings such as hospitals and long-term-care facilities, outbreak-control measures and antiviral chemoprophylaxis are recommended when two cases of laboratory-confirmed influenza are identified in persons on the same ward or unit within 72 hours of each other. In order to prevent transmission, antiviral chemoprophylaxis should be given as soon as possible to all exposed residents or patients regardless of influenza-vaccination history, including those who are on the same ward or unit of the outbreak. Antiviral chemoprophylaxis may also be given to staff who are unvaccinated or who recently received the vaccine (it takes 2 weeks to develop a protective antibody response). Empiric antiviral therapy should be given as soon as possible to any individual with symptoms of active infection during an influenza outbreak.1,7
Follow-up and Monitoring
Patients who do not improve after 2 to 3 days of antiviral treatment should be evaluated for an alternative diagnosis. As previously stated, clinicians should evaluate for bacterial coinfection in patients who worsen after initial improvement with antiviral therapy or in those without clinical improvement after 3 to 5 days of antiviral therapy. Testing for resistance may be considered in patients who develop laboratory-confirmed influenza while taking a neuraminidase inhibitor and in those who do not improve with neuraminidase-inhibitor treatment and have evidence of persistent viral replication.1
The Pharmacist’s Role
Pharmacists can play an integral role in the prevention of influenza virus infection by advocating for annual vaccination and educating clinicians about the appropriate use of chemoprophylaxis in both outpatient and institutional settings. Additionally, pharmacists should work collaboratively with clinicians to select the most appropriate antiviral regimen for a patient infected with influenza, and they can ensure that anti-influenza medications are readily available and counsel patients about side effects of agents and the management of household contacts. Pharmacists are in a key position to actively work with patients and other healthcare providers to ensure the proper management of seasonal influenza.
REFERENCES
1. Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68:e1-e47.
2. CDC. 2018-2019 U.S. flu season: preliminary burden estimates. www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm. Accessed April 22, 2019.
3. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices–United States, 2018-19 influenza season. MMWR Recomm Rep. 2018;67:1-20.
4. CDC. The flu season. www.cdc.gov/flu/about/season/flu-season.htm. Accessed April 23, 2019.
5. CDC. Flu symptoms & complications. www.cdc.gov/flu/symptoms/symptoms.htm. Accessed April 23, 2019.
6. CDC. Diagnosing flu. www.cdc.gov/flu/about/qa/testing.htm. Accessed April 23, 2019.
7. CDC. Influenza antiviral medications: summary for clinicians. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed April 23, 2019.
8. Tamiflu (oseltamivir) package insert. South San Francisco, CA: Genentech, Inc; December 2018.
9. Uyeki T. A step forward in the treatment of influenza. N Engl J Med. 2018;379:975-977.
10. Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2018–2019. Pediatrics. 2018;142(4):e20182367.
11. Xofluza (baloxavir marboxil) package insert. South San Francisco, CA: Genentech, Inc; October 2018.
12. Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379:913-923.
To comment on this article, contact rdavidson@uspharmacist.com.






