US Pharm. 2015;40(5):40-44.
ABSTRACT: Lead toxicity is a major public health concern because it can cause cognitive, behavioral, and motor problems in children. In most cases, exposure can be prevented or at least minimized and eliminated. There are four available agents used to treat lead toxicity: dimercaprol, CaNa2EDTA, succimer, and D-penicillamine. Pharmacists can play an important role in education, treatment, and monitoring of children with lead toxicity. The key to reducing the public burden of lead poisoning is prevention and education.
Although lead is a natural substance, lead toxicity has been a public health concern for over 25 years because it has been linked to cognitive, behavioral, and motor problems in children. The majority of exposures come from two sources: gasoline and paint. Exposure can occur with inhalation or ingestion and, once someone is exposed, it can take years to eliminate lead from the body.1 Even minimal concentrations of lead can cause cognitive defects.
BLOOD LEAD LEVELS
In the past, the CDC designated ≥10 mcg/dL as the blood lead level (BLL) of concern.1 However, there has been much research showing that even lower values can cause cognitive and behavioral deficits. A major study followed children born in 1994 or 1995 for 5 years, monitoring their intelligence quotient (IQ) and BLL.1 In the overall population, it was found that for each 10 mcg/dL increase in BLL, there was a loss of 4.6 IQ points. However, when stratified to include only children whose BLL remained below 10 mcg/dL for all 5 years, the effect on IQ was much greater, at 7.4 points lost. This shows that the relationship between IQ score and BLL is nonlinear and that there is a great decline initially at BLL up to 10 mcg/dL.2 This study, along with 22 other published reports, was used by the CDC in determining that no level of lead exposure is safe.1
In January 2012, the CDC eliminated the use of ≥10 mcg/dL as the BLL of concern. Instead, the CDC looked at BLL percentiles from the National Health and Nutrition Examination Survey (NHANES).3 The CDC conducts NHANES every 2 years and collects health data on U.S. children aged 1 to 5 years.4 The CDC’s new reference value is the 97.5th percentile of the NHANES-generated BLL distribution in children aged 1 to 5 years. This level is currently 5 mcg/dL, which will be reevaluated every 4 years using the last two sets of NHANES data.3
Prevalence
Rates and severity of lead poisoning have been declining with the increase in public health initiatives.4 In the 1970s, the United States banned lead in gasoline, which decreased air emissions and the settling of lead in soil.5 This had a substantial effect on BLLs in children aged 1 to 5 years. From 1976 to 1980, before this legislation took full effect, 88% of children had a BLL ≥10 mcg/dL, with the average BLL being 15 mcg/dL. The average BLL decreased to 3.6 mcg/dL in 1988-1991 and then to 1.9 mcg/dL in 1999-2002. The most recent statistics from 2007-2010 show a decline to 1.3 mcg/dL of average BLL, and only 0.8% of children aged 1 to 5 years with a BLL ≥10 mcg/dL.4 Public health initiatives have had a major impact on reducing exposure to lead, and every NHANES cycle has shown a statistically significant decrease in BLL.4
With gasoline being lead-free for over 35 years, the main source of lead poisoning is deteriorating lead paint. As paint chips off the wall, it can form dust in the building and also spread to nearby soil. Homes built prior to 1950 routinely present as a lead hazard.6 Even though lead paint was banned in the 1970s, many older homes are a source of exposure. In 1998, 25% of homes with a child <6 years old had significant amounts of lead paint, lead dust, or bare soil with significant lead concentration. Traditionally, lead exposure is associated with eating paint chips or chewing on windowsills. While these behaviors do result in higher BLLs, just living in a house with lead paint can cause children to have a BLL of 20 mcg/dL.5 Certain populations are more at risk. Children enrolled in Medicaid, those from poorer families, and African-American children had a higher average BLL than their counterparts in the 2007-2010 NHANES.4
SCREENING
All Medicaid-enrolled children are required to receive a BLL test at ages 12 and 24 months regardless of their risk of lead exposure.3 It is also a Healthcare Effectiveness Data and Information Set (HEDIS) measure for healthcare providers to perform these screenings at 12 and 24 months. If a child misses a scheduled screening, performing a BLL anytime before 72 months is recommended. Many areas have local screening guidelines based on their area’s lead exposure rates. The CDC recommends that healthcare providers follow local guidance but not neglect certain populations at higher risk. Newly immigrated or internationally adopted children should be screened when they arrive in the U.S. In addition, infants born to women with BLLs ≥5 mcg/dL are at higher risk and require routine screening.3
CLINICAL CHARACTERISTICS
The presentation of children with elevated BLLs has changed through the years. Prior to routine BLL screenings, children would present based on symptoms. However, symptoms are not always apparent until BLLs reach 40 mcg/dL, and are nonspecific, including abdominal pain, vomiting, constipation, change in appetite, and irritability. Children typically did not present until their levels were much higher and they were experiencing severe symptoms such as neurologic defects or acute encephalopathy. Encephalopathy was characterized by lethargy, ataxia, seizures, papilledema, and even coma. These kinds of symptoms were associated with a BLL between 70 and 150 mcg/dL and required immediate hospitalization and treatment.6
Today, elevated BLLs are mostly discovered through routine screenings. Most children found to have elevated BLLs are asymptomatic, with a BLL between 10 and 30 mcg/dL.6 It is essential that healthcare providers not equate the absence of symptoms with the absence of toxicity. Due to public health initiatives, current BLLs in the U.S. do not reach the heights of the past, yet damage can occur even at low BLLs, and initiation of treatment is imperative.6
TREATMENT
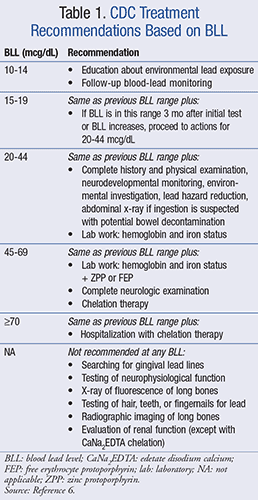
The prevention of further exposure is the foundation of lead-poisoning treatment. The healthcare provider should talk with the patient about environmental exposures to lead and how to reduce these exposures. Treatment is then stratified based on venous BLL. TABLE 1 shows a treatment algorithm based on the CDC guidelines.6 For those with a BLL <19 mcg/dL, education and removal of the cause of exposure are the main concerns. Those with a BLL between 10 and 19 mcg/dL are asked to follow up with blood-lead monitoring. If the level increases or continues to be 15 to 19 mcg/dL 3 months later, or if the initial test was between 20 and 44 mcg/dL, they should proceed to further testing. These patients should have a physical examination and report a complete history. An environmental investigation is needed, and steps to reduce lead exposure should be taken. The patient should be monitored for neurodevelopmental changes and iron status. If lead ingestion is suspected, an abdominal x-ray and potential bowel decontamination should be performed. A BLL ≥45 mcg/dL requires the initiation of outpatient chelation therapy, and a BLL ≥70 mcg/dL requires hospitalization and chelation therapy.6
Iron Deficiency
Patients with high levels of lead are often found to be iron-deficient. Iron deficiency is the most common nutritional deficiency in infants and young children.7 Both lead poisoning and iron deficiency are associated with low socioeconomic status, poor nutrition status, and young age. It is hard to know if these two abnormalities are related or confounded.7
In a cross-sectional study, children were stratified by high, medium, or low lead exposure.8 Those who were iron-deficient had significantly higher BLLs than those who were iron-replete. However, this study was not longitudinal and therefore could not show causation. There could not be certainty as to which came first, the lead toxicity or the iron deficiency. Another study tried to address this question by following children at two doctor visits.9 At each visit, the child’s iron and lead status were evaluated. Those who were iron deficient at their first visit were at risk for elevated BLL at their second visit four to five times more than the children who had normal iron levels initially.9
This relationship has also been studied kinetically. In two studies, it was found that a greater amount of lead was absorbed by iron-deficient animals than by iron-replete animals.7 This is thought to be because iron and lead go through the same receptor for absorption. However, there are studies that do not support this association.6,9 It is still an area of controversy, and further studies are needed to understand the true relationship between iron and lead. The CDC guidelines recommend that children at high risk for lead exposure be tested for iron deficiency and provided with supplementation if needed.3
Chelation Therapy
Chelation therapy works by binding to lead in the blood and soft tissues and creating a compound that can be excreted in the bile and urine. It should only be administered with oversight by a specialist. Local lead-poisoning prevention programs or poison control centers should have a list of qualified practitioners.6 It has been found that chelation in children with a BLL <45 mcg/dL will decrease their BLL, but it will not improve neurodevelopmental test scores.7 Therefore, chelation therapy is usually reserved for patients with a BLL >45 mcg/dL. Eliminating the source of lead exposure may be just as effective at reducing blood levels as chelation therapy in moderately exposed children, based on a study with edetate disodium calcium (CaNa2EDTA).10
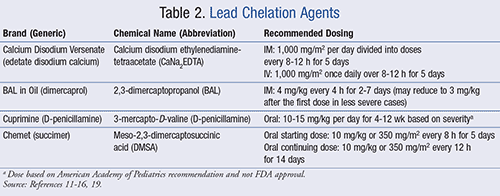
Lead has a high volume of distribution and an extremely long half-life.1 After initial exposure to lead, the BLL rises quickly and then gradually diffuses into other compartments of the body. In children, 70% of lead is stored in the bones.1 Lead can concentrate in the bones for years; therefore, it is extremely difficult to estimate the total body burden based on BLL. Chelating agents remove lead from the blood and soft tissues. It can take years for the deeply stored lead in the bones to move into the circulation and be removed.11 There are currently two parenteral (dimercaprol and CaNa2EDTA) and two oral (succimer and D-penicillamine) agents available, which are listed in TABLE 2.11-16
Dimercaprol: Dimercaprol was created as a chelation agent for Lewisite, an arsenic chemical weapon; thus the name British anti-Lewisite (BAL). It forms a 2:1 chelate with lead that is then excreted in the bile and urine. This agent must be administered IM because of solubility characteristics, and usually is preceded with an antihistamine to reduce adverse effects.11 Common adverse effects include hypertension and tachycardia proportional to the dose administered. About 30% of children experience a fever during treatment. In an acidic environment, the lead-dimercaprol complex can break down and cause kidney damage.12 Some sources recommend alkalization of the urine to protect the kidney during therapy.
Dimercaprol is contraindicated in patients with a peanut allergy or hepatic insufficiency. Patients deficient in glucose-6-phosphate dehydrogenase may experience hemolysis, and those at high risk for hemolysis should be screened prior to therapy. The use of iron supplements is not recommended during treatment because BAL can form a toxic complex with the iron. Although this medication is very old and is associated with adverse effects, it is the drug of choice for severe lead toxicity (BLL >70 mcg/dL).11
Edetate Disodium Calcium: CaNa2EDTA can be administered either IM or IV with a total daily dose of 1,000 mg/m2.13 The IM dose should be administered with procaine or lidocaine to decrease injection site pain, and the total daily dose is divided into IM injections every 8 to 12 hours. IV administration should be diluted in 5% dextrose or normal saline and given slowly to reduce adverse effects. CaNa2EDTA is incompatible with 10% dextrose and Lactated Ringer’s solution.13 The concentration should be <0.5% to avoid phlebitis.11 The total daily IV dose can be infused over a period of 8 to 12 hours. Treatment is given for 5 days, then is stopped for 2 to 4 days to allow the lead to redistribute. A second course of 5 days is then initiated in most cases. The number of courses is determined by the severity of the BLL and the adverse effects of the chelation therapy.13
It is important that sodium EDTA not be substituted for CaNa2EDTA. Doing so may lead to hypocalcemia and cardiac instability. CaNa2EDTA not only binds lead, but also has an affinity for zinc. With prolonged use, it has been associated with zinc deficiency. However, routine use does not require zinc supplementation, and supplementation could decrease its effectiveness.11
CaNa2EDTA has been found to increase lead concentrations in the central nervous system and cause encephalopathy.11 After a single dose of CaNa2EDTA, urinary lead levels increase, blood levels decrease, and brain levels increase significantly due to redistribution of lead from soft tissues into the brain.13 Its use alone is not recommended in patients with encephalopathy or with a BLL >70 mcg/dL. It should be given with dimercaprol to decrease lead redistribution into the brain.11
Dimercaprol + Edetate Disodium Calcium: It has been found that treatment with dimercaprol prior to CaNa2EDTA therapy may decrease the risk for encephalopathy due to the CaNa2EDTA.11 When they are used in combination, BLLs are decreased at a faster rate. However, this may have little clinical significance, since postchelation BLLs are no different from those with either therapy alone. Combination therapy has been shown to increase side effects, mainly elevated liver enzymes and vomiting. However, due to the decreased risk of encephalopathy, this combination is recommended in patients with a BLL >70 mcg/dL.11
The combination dose for acute lead encephalopathy is 4 mg/kg of dimercaprol IM every 4 hours, with the first dose being given alone.12 Starting with the second dose, CaNa2EDTA is added with a total daily dose of 1,000 mg/m2 given every 8 to 12 hours IM or once daily IV over an 8- to 12-hour interval. For less severe toxicity, the dimercaprol dose can be decreased to 3 mg/kg after the first dose. Treatment should continue for 2 to 7 days depending on clinical response (decreased BLL, reversal of the hematologic effects of lead, and an increased concentration of lead in the urine).11
Succimer: Succimer is a derivative of dimercaprol that can be administered orally. It has a specific affinity for lead and less affinity for iron, zinc, and calcium. An advantage to dimercaprol is that succimer does not have a toxic reaction when coadministered with iron.11 Animal studies suggest that succimer is not likely to precipitate encephalopathy as CaNa2EDTA does. Adverse effects in children include 12% gastrointestinal complaints, 5% malaise, and 4% transient elevated hepatic enzymes.11 Other side effects include a decreased hemoglobin level, reversible neutropenia, and hypersensitivity reactions. Despite these, succimer is much better tolerated than the two parenteral chelates. It can be used in either the outpatient or inpatient setting, and adherence is key to success.11
The starting dose is 10 mg/kg or 350 mg/m2 every 8 hours for 5 days.14 Then the frequency is reduced to 10 mg/kg or 350 mg/m2 every 12 hours for an additional 2 weeks. This is a total treatment of 19 days. A second treatment can be given 2 weeks later, if needed, based on the BLL. Succimer is supplied in 100 mg capsules that can be opened and sprinkled on soft food or swallowed whole.14
D-Penicillamine: This chelate is not FDA-approved for lead poisoning. It is approved for the treatment of Wilson disease, cystinuria, and rheumatoid arthritis.15 D-penicillamine is usually used as a follow-up treatment to CaNa2EDTA or dimercaprol to stop the BLL from rebounding after parenteral treatment.11 An advantage of this treatment is that it can be taken orally. Food reduces bioavailability by 35%, and antacids decrease bioavailability by 66%.11 This drug must be given on an empty stomach, at least 1 hour before meals or 2 hours after meals.15
The dosing of this medication is based on empirical data and not FDA approval; therefore, there are many different recommendations. When D-penicillamine was first studied, doses as high as 100 mg/kg were used. More recently, smaller doses have been found to be effective.11 The American Academy of Pediatrics recommends a dosage of 10 to 15 mg/kg per day for 4 to 12 weeks.16 The Harriet Lane Handbook published by Johns Hopkins Hospital recommends 25 to 35 mg/kg/day in divided doses, starting at 25% of this dosage and increasing to the full dosage over 2 to 3 weeks.17 Canadian labeling recommends 30 to 40 mg/kg/day as a single dose or in two divided doses, with a maximum of 750 mg/day.18 A toxicology expert should be consulted before initiation of this drug because current dosing is based on limited empirical data, and the drug may cause serious adverse effects.6 In the past, D-penicillamine was synthesized by degrading the antibiotic penicillin, causing a cross-sensitivity reaction in patients allergic to penicillin. However, D-penicillamine is now made synthetically and the chance of cross-sensitivity is much lower.15
Adverse effects include reversible leukopenia, mild thrombocytopenia, and eosinophilia in 10% to 20% of children.11 Angioedema, urticaria, or maculopapular rash may develop in 0.5% to 1% of children, requiring discontinuation of therapy. Due to adverse events, D-penicillamine should be used as a third-line treatment for lead toxicity. Patients who do not tolerate succimer and CaNa2EDTA yet require further treatment may be candidates for D-penicillamine.11
PHARMACIST’S ROLE
Pharmacists have a unique role in the healthcare system because of their accessibility to and close contact with the community. This is an opportunity to recognize signs and symptoms of lead toxicity in children and take the appropriate steps to refer children for screening. Pharmacists are also able to answer questions that patients may have about lead toxicity and counsel on ways to decrease lead exposure. Pharmacists can also participate in public health campaigns and community education about lead toxicity. When it comes to chelation therapy, pharmacists are able to make recommendations and monitor treatment. For the inpatient setting, pharmacists can ensure that chelates are prepared and administered properly. For the outpatient setting, it is essential for pharmacists to counsel patients on the correct use of chelates, the importance of adherence, and potential adverse effects.
CONCLUSION
With the increase in public health initiatives and the removal of lead from gasoline and paint, the prevalence of lead toxicity has greatly decreased. However, it has recently been discovered that even lower lead concentrations can have a negative effect on cognitive development. No amount of lead exposure is safe, and the healthcare system must therefore be vigilant in screening children. Education and prevention are key to reducing the impact of lead on children. Treatment algorithms focus on reducing exposure and initiating chelation therapy at high lead levels. Pharmacists can play an important role in identifying those at risk, educating the public, and monitoring chelation therapy. Each NHANES cycle has seen a decline in average BLL. Public health initiatives and a unified healthcare effort will not stop until lead exposure is eliminated.
REFERENCES
1. Binns HJ, Campbell C, Brown MJ, et al. Interpreting and managing blood lead levels of less than 10 µg/dL in children and reducing childhood exposure to lead: recommendations of the Centers for Disease Control and Prevention Advisory Committee on Childhood Lead Poisoning Prevention. Pediatrics. 2007;120:e1285-e1298.
2. Canfield RL, Henderson CR, Cory-Slechta DA, et al. Intellectual impairment in children with blood lead concentrations below 10 µg/dL. N Engl J Med. 2003;348:1517-1526.
3. Advisory Committee on Childhood Lead Poisoning Prevention Recommendations. Low level lead exposure harms children: a renewed call of primary prevention. www.cdc.gov/nceh/lead/ACCLPP/activities.htm. Accessed January 26, 2015.
4. CDC. Blood lead levels in children aged 1-5 years—United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2013;62:245.
5. American Academy of Pediatrics. Lead exposure in children: prevention, detection, and management. Pediatrics. 2005;116(4):1036-1046.
6. Roberts JR, Reigart JR. Medical assessment and interventions. In: Managing Elevated Blood Lead Levels Among Young Children: Recommendations from the Advisory Committee on Childhood Lead Poisoning Prevention. Atlanta, GA: CDC; 2002.
7. Sargent J. Nutritional assessment and interventions. In: Managing Elevated Blood Lead Levels Among Young Children: Recommendations from the Advisory Committee on Childhood Lead Poisoning Prevention. Atlanta, GA: CDC; 2002.
8. Bradman A, Eskenazi B, Sutton P, et al. Iron deficiency associated with higher blood lead in children living in contaminated environments. Environ Health Perspect. 2001;109:1079-1084.
9. Write RO, Tsaih SW, Schwartz J, et al. Association between iron deficiency and blood lead level in a longitudinal analysis of children followed in an urban primary care clinic. J Pediatr. 2003;142:9-14.
10. Ruff HA, Bijur PE, Markowitz M, et al. Declining blood lead levels and cognitive changes in moderately lead-poisoned children. JAMA. 1993;269:1641-1646.
11. American Academy of Pediatrics Committee on Drugs. Treatment guidelines for lead exposure in children. Pediatrics. 1995;96:155-160.
12. BAL in Oil (dimercaprol) package insert. Lake Forest, IL: Akorn; April 2012.
13. Calcium Disodium Versenate (edetate calcium disodium injection) package insert. Northridge, CA: 3M Pharmaceuticals; September 2007.
14. Chemet (succimer) package insert. Seymour, IN: Kremers Urban Pharmaceuticals Inc; February 2013.
15. Cuprimine (penicillamine) package insert. Lawrenceville, NJ: Aton Pharma; August 2012.
16. Chandran L, Cataldo R. Lead poisoning: basics and new developments. Pediatr Rev. 2010;31:339-406.
17. Nayak Z, Ronda J. Poisonings. In: Johns Hopkins Hospital; Engorn B, Ferlage J. The Harriet Lane Handbook. 20th ed. Philadelphia, PA: Elsevier; 2015:19-26.
18. Repchinsky C, et al. Cuprimine in Compendium of Pharmaceuticals and Specialties. 9th ed. Ottawa, ON: Canadian Pharmacists Association; 2009:644.
19. Tarrago O, Demers R. Case Studies in Environmental Medicine: Lead Toxicity. Atlanta, GA: Agency for Toxic Substance and Disease Registry; 2010.
To comment on this article, contact rdavidson@uspharmacist.com.





