US Pharm. 2016;41(4):22-26.
ABSTRACT: Lyme disease, the most common tick-borne infection in the United States, is usually caused by the bacterium Borrelia burgdorferi. While the typical manifestation of early disease is a skin lesion called erythema migrans, progression to more severe neurologic and cardiac sequelae can occur. When symptoms are identified early, there is a high cure rate with antimicrobial treatment. Most important, the disease is preventable with prompt identification and removal of all ticks and with other measures such as appropriate clothing and use of insecticides. Pharmacists can play a role in assisting in prevention and treatment of early disease.
Lyme disease is the most common tick-borne bacterial infection caused by the spirochete Borrelia burgdorferi in North America and Europe, with approximately 300,000 cases annually in the United States alone.1 It was first recognized in 1976 with multiple reports of rheumatoid arthritis in children in and around Lyme, Connecticut.2 Affected individuals recalled a tick bite followed by a red, expanding rash several weeks before the arthritis onset. Researchers noted similarities regarding location and season of peak occurrence, which led them to believe it was transmitted by an arthropod vector, specifically of the Ixodes tick genus.3,4
In 1981, Burgdorfer and colleagues discovered a spirochetal bacterium (B burgdorferi ) which is the same organism found in Ixodes cultured from Lyme disease patients.2 Recently, a newly identified Lyme disease–causing species, Borrelia mayonii, has been detected in acutely ill patients residing in the upper Midwest—specifically in Minnesota, Wisconsin, and North Dakota—who exhibited symptoms after being bitten by Ixodes scapularis ticks.5
Transmission
In the U.S., B burgdorferi is trans-mitted by I scapularis (deer tick) and Ixodes pacificus (western black-legged tick).6,7 Fortunately, I pacificus ticks are rarely infected by B burg-dorferi and account for only about 1% of actual patient infections.2,7,8 I scapularis ticks are the principal vector, as they are ubiquitous in the northeastern and north-central states due to the abundance of the white-footed mice they feed on.2
There are three separate develop-mental stages within the two-year Ixodes life cycle: larva, nymph, and adult. Female adult ticks lay eggs that hatch during the summer, resulting in larval-staged ticks that feed on white-footed mice in the late summer. Nymphs most commonly transmit B burgdorferi to humans by feeding on white-footed mice and humans in the late spring and early summer. Adult ticks feed in the fall on larger mammals, especially deer, and may also transmit B burgdorferi to humans.2,7
B burgdorferi spends most of its natural cycle in the midgut of the tick. During feeding, the tick’s body slowly enlarges.7,9 In order to transmit infection, the tick requires at least 36 hours of attachment with an exponential increase in the infection risk after 48 to 72 hours.9,10 There are no known cases of transmission through contact with infected blood nor credible evidence of transmission through sexual contact, semen, urine, or breast milk.7
Risk Factors
Although there is a geographic risk within endemic areas, cases have been reported from almost every state in the U.S.7 However, 95% of all cases in 2012 were reported in 13 states; namely Connecticut, Delaware, Maine, Maryland, Massachusetts, Minnesota, New Hampshire, New Jersey, New York, Pennsylvania, Vermont, Virginia, and Wisconsin.11 Ticks that transmit B burgdorferi are susceptible to desiccation and need constant high relative humidity; therefore, infection risk is greatest in the late spring and summer.9,12 Ticks tend to reside in the fringe areas between lawns and woods and in overgrown brush and can be carried by animals onto lawns and into houses by pets.9 A national study of tick endemic areas found that certain occupations and hobbies can also increase infection risk, as in forestry workers, farmers, and hikers.7
Lyme Disease Stages and Diagnosis
Lyme disease progresses through several stages, manifesting as different symptoms depending on duration of infection and location of affected area. Stage 1, known as “early localized Lyme disease,” develops days to weeks after a person has become infected. The most common presentation is a uniformly red rash known as erythema migrans that develops from the initial tick bite and deposition of B burgdorferi.13 In many cases, the person may not even notice any symptoms.
If Lyme disease is not diagnosed or treated while early symptoms are present or if the person did not experience any early symptoms, the infection will progress to stage 2, “early disseminated Lyme disease,” as the bacteria begin to spread throughout the body.1,9 Clinical manifestations include flulike symptoms, with or without the rash. As the infection spreads, the rash continues to expand and appear on other body parts. The disease also may begin to affect the joints and nervous system within weeks to months after the initial infection, causing weakness or numbness of the arms and legs.
Months to years after, a person may advance to stage 3, which is “late disseminated Lyme disease,” indicating that the bacteria have spread throughout the entire body, and may present with muscle and joint pain.9 While long-term joint inflammation, also known as Lyme arthritis, and heart problems are common, damage to the nervous system is also possible, causing speech irregularities, cognitive impairment, and facial paralysis. Consequently, when these symptoms occur, a patient has fully progressed to the last stage of Lyme disease.
Because Lyme disease can present as three distinct phases, the results of diagnostic laboratory tests may vary based on disease acuity and progression. Thus, signs and symptoms as well as the history of tick exposure should be the primary source of Lyme disease diagnosis, as recommended by the CDC.9 It is important to note that for patients presenting solely with erythema migrans, the clinical diagnosis of Lyme disease may be considered even in the absence of any laboratory testing.1 However, laboratory testing is available for diagnosis as well, though not routinely recommended, in patients with erythema migrans due to low sensitivity during the acute or early stages.6 Laboratory confirmation with serologic testing may be of value in patients with other manifestations of Lyme disease where diagnosis remains uncertain.1 The diagnosis of Lyme disease consists of a two-tiered serologic testing, which includes an enzyme-linked immunosorbent assay (ELISA) followed by a Western blot,9 and is only recommended by the CDC for symptomatic patients.
Clinical Manifestations and Treatment
Current guidelines from the Infectious Diseases Society of America (IDSA) associate treatment with clinical manifestations.1 Thus, it is essential for healthcare professionals, including pharmacists, to be able to identify the early and late signs and symptoms of Lyme disease. Chemoprophylaxis may be considered only in patients who meet all of the following criteria: 1) the tick has been attached for ≥36 hours; 2) the tick is identified as an adult or nymphal I scapularis tick; 3) initiation of prophylaxis with antibiotics is within 72 hours of tick removal; 4) the B burgdorferi infection rate is ≥20% in the local area; and 5) there are no contraindications to doxycycline.1 For patients >8 years old requiring prophylaxis, a one-time oral dose of doxycycline (200 mg in adults or 4 mg/kg up to 200 mg/day in children) is recommended.1 In the event that an infection does develop, antibiotic treatment has been proven to be highly effective with cure rates of about 90%.6
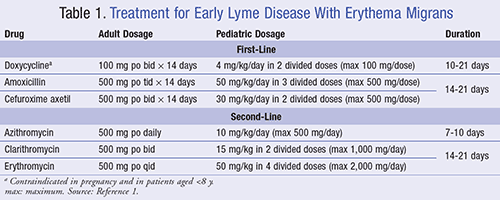
Erythema migrans will expand gradually over a period of days reaching up to 30 cm in diameter with varying appearance. Some eruptions may be homogeneous in color, while others may have a prominent targetlike appearance with or without pustules at the center.14 For adults presenting with erythema migrans and lacking any neurologic findings or advanced atrioventricular (AV) heart block, treatment options include a 14-day oral regimen of either doxycycline, amoxicillin, or cefuroxime (see TABLE 1).1 For pediatric patients, the treatment regimen and duration are identical with the exception of weight-based dosing (TABLE 1).1 For those with allergies or any contraindications with the first-line agents, macrolides may be used as a second-line option, although efficacy is lower with these agents.1
Early neurologic Lyme disease may develop and most commonly manifests with cranial neuropathy, specifically 7th nerve palsy (formerly called Bell’s palsy). It involves the paralysis of any facial nerve and can even develop in the absence of erythema migrans.15 In late Lyme disease, neurologic symptoms may progress to meningitis and encephalomyelitis.16 If a patient shows symptoms of central nervous system involvement, then a lumbar puncture (LP) may be performed.1 If an LP is not indicated or is negative, symptomatic patients may be treated with the same approach as for those who present with erythema migrans.1 If a patient has both clinical and laboratory evidence supporting neurologic involvement, then ceftriaxone is the drug of choice for treatment (see TABLE 2).1
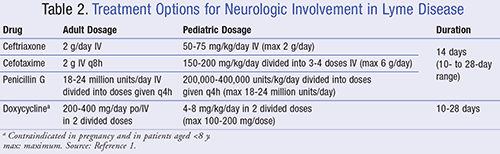
Lyme carditis arises when B burgdorferi spirochetes invade cardiac tissues, although the occurrence has only been reported in about 1% of cases from 2001 to 2010.17 The predominant manifestation is AV heart block, although atrial fibrillation, myopericarditis, and, rarely, acute heart failure may occur.14 The IDSA recommends treatment with either IV or oral antibiotics for patients with AV heart block and/or myopericarditis.1 Symptomatic patients should be hospitalized and treated with ceftriaxone (see TABLE 2 for dosing) for at least 14 days (range 14-21 days).1 Upon hospital discharge, IV therapy should be switched to an oral antibiotic as listed in TABLE 1.1
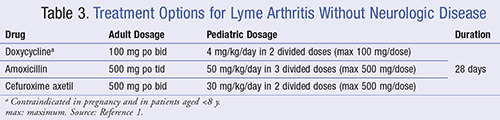
Lyme arthritis manifests subsequently, causing joint pain and swelling, primarily in the knee.18 For symptomatic patients without neurologic disease, the IDSA recommends a 28-day treatment with either doxycycline, amoxicillin, or cefuroxime, as listed in TABLE 3.1 Patients with Lyme arthritis with neurologic disease, as supported by objective evidence, should receive the same treatment as those with Lyme meningitis (see TABLE 2).1 Those who have persistent or recurrent joint swelling after receiving a full course of oral antibiotics may be retreated with an additional course of oral antibiotics for 4 weeks or ceftriaxone 2 g IV daily for 2 to 4 weeks.1 Providers should note that inflammation tends to subside slowly after treatment; thus, it is reasonable to wait a few months before considering retreatment.1 Even after retreatment, recalcitrant patients should follow-up with a rheumatologist and may manage their symptoms with nonsteroidal anti-inflammatory drugs (NSAIDs), intra-articular corticosteroid injections, or disease-modifying antirheumatic drugs (DMARDs).1
Lastly, patients may present with unexplained chronic subjective symptoms even after conventional antibiotic treatment, which defines chronic Lyme disease, Lyme encephalopathy, or posttreatment Lyme disease syndrome (PTLDS). PTLDS describes the phenomenon wherein symptoms persist for many months to years after completion of appropriate antibiotic treatment. Although it is unclear why symptoms persist, there is a belief that it results from residual damage of tissues or infection from lingering bacteria.19 It is more common for patients to continue having subjective manifestations such as joint and muscle pain, paresthesias, and fatigue rather than typical late objective manifestations.20 Nonetheless, there is no convincing biologic evidence to warrant these chronic symptoms or to prove the benefit of additional antibiotics after the initial course. Therefore, patients with nonspecific symptoms but lacking objective signs of disease should not receive antibiotic therapy, since the symptoms are self-limiting and resolve over time.6
Prevention
Pharmacists can provide guidance in selecting insect repellants proven safe and effective and counseling on appropriate attire when potentially exposed to endemic areas.20 The best available method, according to current guidelines, is to avoid tick-infested areas, especially during the summer.1 It is also recommended to wear light-colored protective clothing in order to identify the black-colored ticks, with shirts tucked into pants and pants tucked into socks. Clothes should be washed and dried at high temperatures to remove any residual ticks.1,9
Permethrin is approved by the Environmental Protection Agency (EPA) to spray on clothing to kill ticks on contact.20 Since the tick has to feed for ≥48 hours to transmit the bacteria, the best practice is to bathe or shower as soon as possible after exposure and to carefully check for ticks on clothes and skin. Proper removal of an attached tick requires using a fine-tip forceps to lift directly up to detach the tick from the skin.9 It is advised not to attempt to remove the mouth part of the tick if it remains, and to clean the affected area with antiseptic.1,9
DEET (N,N-diethyl-meta-toluamide) and picaridin are the only two insect repellents registered with the EPA that can be applied to exposed skin and clothing. DEET is safe for patients of all ages. However, neurotoxicity may occur with excessive use. Therefore, children should not be allowed to use it without adult supervision. It should not be applied in areas that can increase absorption such as abrasions or wounds. To avoid contact with the eyes and mouth, pump or aerosol spray formulations should be applied to the hands first, then to the face.1,20 In a review of agents for arthropod bite prevention, DEET is considered the gold standard due to its wide use and broad-spectrum protection. The CDC recommends using products containing up to 50% of DEET, since the duration of activity does not increase with higher concentrations.21,22 As an alternative, picaridin works similarly to DEET and is purported to have less neurotoxicity.22
Conclusion
Since Lyme disease is the most common tick-borne infection in the U.S., it is increasingly important for pharmacists to be involved in its prevention and management. As frontline healthcare providers, pharmacists can offer counseling on potential risks in exposure to endemic areas and on proper selection and application of insecticides for prevention. As they are sought out for acute relief, pharmacists may assist in early identification of erythema migrans, further preventing sequelae. With the various manifestations of infection, pharmacists also assist in navigating the complex process and play a role in appropriate antimicrobial selection and dosing as well as monitoring of response to therapy. It is clear that pharmacists can have a significant impact in the management of Lyme disease.
REFERENCES
1. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089-1134.
2. Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113(8):1093-1101.
3. Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum. 1977;20:7-17.
4. National Institute of Allergy and Infectious Diseases. A history of Lyme disease, symptoms, diagnosis, treatment, and prevention. July 1, 2015. www.niaid.nih.gov/topics/lymedisease/Pages/History.aspx. Accessed November 16, 2015.
5. Pritt BS, Mead PS, Johnson DK, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016 Feb 5; Epub ahead of print. www.thelancet.com/journals/laninf/article/PIIS1473-3099(15)00464-8/abstract. Accessed March 7, 2016.
6. Shapiro ED. Lyme disease. N Engl J Med. 2014;370:1724-1731.
7. Meade PS. Epidemiology of Lyme disease. Infect Dis Clin N Am. 2015;29:187-210.
8. Eisen L, Lane RS. Vectors of Borrelia burgdorferi sensu lato. In: Gray JS, Kahl O, Lane RS, et al, eds. Lyme Borreliosis Biology, Epidemiology and Control. Wallingford, UK: CABI Publishing; 2002:91.
9. CDC. Lyme disease. Last updated October 27, 2015. www.cdc.gov/lyme. Accessed November 14, 2015.
10. Sood SK, Salzman MB, Johnson BJ, et al. Duration of tick attachment as a predictor of the risk of Lyme disease in an area in which Lyme disease is endemic. J Infect Dis. 1997;175:996-999.
11. CDC. Final 2012 reports of nationally notifiable infectious diseases. MMWR Morb Mortal Wkly Rep. 2013; 62:669-682. www.cdc.gov/mmwr/preview/mmwrhtml/mm6233a6.htm. Accessed March 7, 2016.
12. Ostfeld RS, Brunner JL. Climate change and Ixodes tick-borne diseases of humans. Philos Trans R Soc Lond B Biol Sci. 2015;370(1665):1-11.
13. Juckett G. Arthropod bites. Am Fam Physician. 2013;88(12):841-847.
14. Cameron DJ, Johnson LB, Maloney EL. Evidence assessments and guideline recommendations in Lyme disease: the clinical management of known tick bites, erythema migrans rashes and persistent disease. Exp Rev Anti-Infect Ther. 2014;12(9):1103-1135.
15. Stratmoen J. Neurologic complications of Lyme disease: dilemmas in diagnosis and treatment. Neurol Today. 2004;4(4):71-75.
16. Kalina P, Decker A, Kornel E, et al. Lyme disease of the brainstem. Neuroradiology. 2005;47:903-907.
17. Robinson ML, Kobayashi T, Higgins Y, et al. Lyme carditis. Infect Dis Clin North Am. 2015;29(2):255-268.
18. Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. 2015;29(2):269-280.
19. Steiner I. Treating post-Lyme disease: trying to solve one equation with too many unknowns. Neurology. 2003;60:1888-1889.
20. Insect repellents: reducing insect bites. EPA. www2.epa.gov/insect-repellents. Accessed November 20, 2015.
21. Goodyer LI, Croft AM, Frances SP, et al. Expert review of the evidence base for arthropod bite avoidance. J Travel Med. 2010;17:182-192.
22. Dolan MC, Panella NA. A review of arthropod repellents. In: Paluch GE, Coats JR. Recent Developments in Invertebrate Repellents. Washington DC: American Chemical Society; 2011:1-19.
To comment on this article, contact rdavidson@uspharmacist.com.





