US Pharm. 2011;36(10):60-68.
Rheumatoid arthritis (RA) is a chronic auto-immune and progressive inflammatory disorder that affects the synovial joints.1 It is a systemic rather than a joint disease that typically affects the small joints of the hands and feet, usually symmetrically.2,3
RA is three times more prevalent in women than in men and affects about 0.5% to 1% of the worldwide population.1,4 Even though many advances have been made in the management of the disease, the incidence, particularly of cardiovascular mortality, has not shown a great improvement.5,6
Like most chronic inflammatory and degenerative disorders, RA has a complex genetic etiology.7 While the initial trigger is not known, smoking has consistently been shown to increase prevalence, whereas occupational factors such as exposure to asphalt or silica require further investigation.8
Signs and Symptoms
Disease progression results in joint destruction, deformities, and eventually disabilities. Patients complain of redness, warmth, swelling, and prolonged morning stiffness of both the small and large joints. These symptoms may develop suddenly or over weeks or months. Commonly, patients experience sickness as well as weight loss.9 Morning symptoms of RA are a sign of active disease—they are characterized by elevated nocturnal levels of proinflammatory cytokines that are implicated in joint damage. These cytokines are insufficiently suppressed by endogenous cortisol.3 Extra-articular features commonly include malaise, fever, fatigue, weight loss, and anemia.10 More significant inflammatory manifestations may lead to serious pathology such as vasculitis, pericarditis, pleurisy, pleural effusion, pulmonary interstitial fibrosis, peripheral neuropathies, subcutaneous and pulmonary nodules, scleritis, and Sjögren’s syndrome.3,10 Patients with RA are at a greater risk of atherosclerosis as a result of the inflammation, osteoporosis due to reduced joint mobility, and infections because of abnormalities in the immune system.
Owing to the debilitating nature of the disease, it is imperative that a multidisciplinary approach be used for the management of RA, involving pharmacists, physiotherapists, podiatrists, physicians, and even social workers. The primary goal is twofold: to provide symptomatic relief and to reduce bone destruction; the focus should be on the whole body and not just the affected joint.3,11 This article will discuss the options available for both nonpharmacologic and pharmacologic therapy. In some cases, surgery may be required.
Nonpharmacologic Therapy
Nonpharmacologic therapies include exercise, complementary therapies, supplements, diet, and herbs. Exercise regimens such as aquatic therapy, yoga, and tai chi have proven useful in chronic pain management of RA.8 Exercise increases energy levels and reduces functional limitations.9 Patients also have a wide variety of complementary therapies to chose from, including relaxation, meditation, hypnosis, massage, homeopathy, magnet therapy, music therapy, imagery, and therapeutic touch.8
Furthermore, splinting can be used to stretch severely deformed joints, especially in children with RA.9 Massage, cold and heat therapy, and physical therapy all help maintain joint mobility.9
Pharmacologic Therapy
Drug therapy for RA consists of analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs), which are used primarily to relieve pain; disease-modifying antirheumatic drugs (DMARDs), which prevent joint destruction; and corticosteroids, which have an anti-inflammatory effect.
Analgesics: Simple analgesics such as acetaminophen, tramadol, and dihydrocodeine have no anti-inflammatory properties but are useful at controlling the pain and soreness associated with RA.10
NSAIDs: These drugs are slightly stronger than acetaminophen on its own due to their anti-inflammatory properties. Even though NSAIDs are very similar, individuals respond differently to different NSAIDs and hence the choice of NSAID varies between patients.10 The dose of the chosen NSAID should be increased gradually over 1 or 2 weeks to reduce the risk of side effects. Cyclooxygenase-2 (COX-2) inhibitors such as celecoxib are useful in patients at an increased risk of gastrointestinal (GI) symptoms.10
Corticosteroids: The use of corticosteroids is controversial due to the associated adverse effects. Where required, the dose should be kept as low as possible to reduce the risk of adverse effects such as osteoporosis, hypertension, cataracts, infections, and skin fragility. Normal doses of prednisolone are 5 to 10 mg daily but may be increased up to 60 mg. In severe flare-ups, patients have been given IV or intra-articular injections of methylprednisolone.9 Injections are particularly useful in cases where only one or two joints are affected. Long-term, low-dose glucocorticoid therapy is justifiable, especially if the bones are protected for osteopenia, whereas higher doses are normally used as a bridge between treatments and until DMARDs become effective.12
Nonbiological DMARDs: While NSAIDs provide symptomatic relief, they do not halt disease progression. DMARDs, however, have the effect of slowing down the disease, even though they take slightly longer to work than NSAIDs and analgesics.9 Thus, DMARDs interfere with the disease process.4
Contrary to previous guidelines, it is now evident that DMARD therapy is most effective when initiated early on in the disease, since irreversible joint damage occurs in the first 2 years.9 In fact, a recent study comparing DMARDs, glucocorticoids, biologics, and combination treatments concluded that DMARDs should be retained as first-line therapy.13 However, since the effects of DMARDs are not immediate, they should be tried for at least 6 months. If there is no benefit during this time period, a second DMARD may be tried.10 In some cases, combining DMARDs may provide effective therapy.10 TABLE 1 lists the different types of nonbiological DMARDs, with the most commonly used agents being hydroxychloroquine (HCQ), sulfasalazine (SSZ), methotrexate (MTX), and leflunomide.
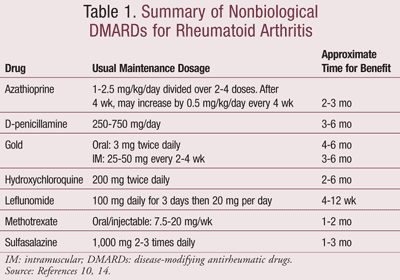
The choice of DMARD varies between patients and is usually dependent on a number of factors including efficacy, convenience of administration, monitoring requirements, cost of medication and monitoring, time until expected benefit, frequency and severity of adverse effect, interactions with other medications the patient is taking, and comorbid conditions.14 First choices for DMARDs include HCQ and SSZ or MTX for patients with highly active disease.14
HCQ may not be as effective as other DMARDs, but it has a significant impact on long-term patient outcomes.10,14 It is also better tolerated and associated with fewer side effects and is therefore useful in milder forms of the disease. Patients may infrequently complain of rash, abdominal cramps, and diarrhea. Beneficial effects are seen between 1 and 6 months.
SSZ may act more quickly than HCQ and may be the drug of choice in many patients who have mild RA. Patients may experience nausea and abdominal discomfort—adverse effects that can be minimized by starting with low doses and gradually increasing. Rare but more serious adverse effects include leucopenia.14
Methotrexate has established itself as an anchor drug in the management of RA due to its favorable efficacy, toxicity profile, and low cost.9,12,14 MTX alleviates pain as well as morning stiffness.9 A weekly low dose has shown to be anti-inflammatory rather than a high dose, which tends to be cytotoxic.12 It takes 3 to 4 weeks for its effects to be seen, and the maximal effect is seen in about 6 months.9 Common adverse effects associated with the use of MTX include oral ulcers, nausea, vomiting, anorexia, increased risk of infection, fatigue, malaise, and infertility. Furthermore, patients should be regularly monitored for liver and renal function, and a complete blood count (CBC) should be carried out every 4 to 8 weeks. MTX can cause liver toxicity, even in doses as low as those used in psoriasis. The U.S. guidelines therefore recommend an initial liver biopsy and then subsequently after each cumulative dose of 1 to 1.5 g, as well as the monitoring of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, bilirubin, and albumin.10
The use of MTX is contraindicated in pregnant women, as it is potentially teratogenic, and in those with impaired renal function, as is the consumption of alcohol in patients taking MTX. Women of childbearing age taking MTX should use contraception.
Various measures can be employed to reduce the toxicity of MTX. Folinic acid neutralizes the immediate toxic effects of MTX on the bone marrow.10 It may be given orally or via injection. Additionally, to prevent the precipitation of MTX or its metabolites in the renal tubules, acetazolamide or sodium bicarbonate is often prescribed to maintain the flow of alkaline urine.
Leflunomide is a suitable alternative to MTX as monotherapy in patients who are either not responding to or cannot tolerate MTX.9,14 Alternatively, leflunomide can be used in combination with MTX in patients who do not obtain a complete clinical response with MTX alone. Common adverse effects include a skin rash, abdominal pain, diarrhea, nausea, reversible hair loss, and an elevation of liver enzymes. Since enterohepatic circulation plays an important role in leflunomide, the drug has a half-life of as long as 2 years. It is very essential to monitor liver function with regular liver tests during leflunomide therapy. For the same reason, the use of leflunomide is contraindicated in patients with obstructive biliary disease, liver disease, viral hepatitis, severe immunodeficiency, and rifampin therapy. As with MTX, leflunomide is a potential teratogen, and women of childbearing age should be advised to use effective contraception. It is important to note that patients taking leflunomide are at an increased risk of infection.9,14
Azathioprine (AZA), an older DMARD, is now rarely used. D-penicillamine is effective but has an inconvenient dosage schedule and is therefore not favored. While intramuscular (IM) gold is effective, patients need to be injected weekly for 22 weeks before the frequency can be decreased. Oral gold, however, is administered more conveniently but takes longer to take effect.14
Biological DMARDs: Biologics are biological response modifiers that target the pathophysiologic processes of RA.15 Initial biological DMARDs included those that block cytokines and tumor necrosis factor (TNF), as well as interleukin receptor antagonists. These have led to the development of newer agents that target alternative pathways such as B-cell depletion and T-cell costimulation agents. Biologics attempt to reduce inflammation, pain, and bone destruction by balancing excess cytokines.9 The body releases natural anti-inflammatory agents, such as the soluble receptor for TNF (sTNFR), to fight the inflammation. sTNFR neutralizes TNF to reduce the inflammatory response. The first biological agent, etanercept, is a genetically engineered version of sTNFR that is now part of a group of drugs known as the TNF inhibitors.9 Following these, newer targets have been established and are listed in TABLE 2. The newer TNF inhibitors, certolizumab and golimumab, neutralize TNF.4
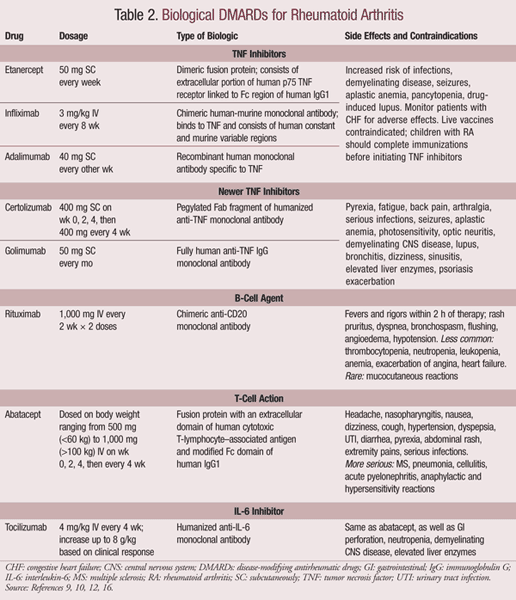
TNF inhibitors are generally recommended for patients with moderate to severe disease who cannot tolerate MTX or have not responded to at least two DMARDs.9 They are either self-administered subcutaneously or given intravenously by a trained medical professional.16 Any irritation at the site of injection can be managed using antihistamines or topical corticosteroids.9
Reports show that bacterial infections, invasive fungal infections, and other opportunistic infections increase with the use of TNF inhibitors. This risk is lower in patients taking etanercept as compared to other drugs. Preinitiation screening procedures have assisted in reducing the number of cases that present with reactivated latent tuberculosis following treatment with TNF inhibitors.16 All TNF inhibitors except etanercept are associated with the development of neutralizing antibodies, which reduce the efficacy of these agents. This reaction can be reduced by combining the TNF inhibitors with MTX.16
All other biologics, including rituximab, abtacept, and tocilizumab, are indicated for moderate-to-severe RA that has not responded adequately to one or more nonbiological DMARDs or TNF inhibitors.16
Surgical Options
Surgery at the affected joints is a last option for patients who do not respond to any of the previously discussed therapies. Due to the nature of the procedure, many patients may choose to live with their symptoms rather than undergo surgery.
Alternative Therapies
A recent review of alternative therapies for RA was not able to conclude whether vitamin E alone; a combination of vitamins A, C, and E; ginger; turmeric; or Zyflamend are sufficient to reduce the recommended doses of NSAIDs or corticosteroids.17
Future Developments
Despite substantial developments in therapies for RA, there has been limited improvement in the mortality of patients with the disease. Since RA has such a close link to genetics, future developments in gene therapy will help to improve customized treatment regimens.7,17 However, more work is still required to understand the contributory mechanisms and determinants in RA in order to improve management and reduce the mortality gap.5 The protein kinase inhibitors, particularly the Janus kinases, spleen tyrosine kinases, and the inhibitors of p38 mitogen-activated protein kinases, are currently areas of great interest.4 Following the success of rituximab, two other B-cell depleting agents, ofatumumab and ocrelizumab, are presently being investigated in the management of RA.4
Role of the Pharmacist
Pharmacists are a valuable centerpiece in counseling patients on how to take their RA medications correctly and in stressing the importance of compliance with their regimen. Furthermore, pharmacists should make themselves available to patients who may have concerns regarding their current RA therapy.
REFERENCES
1. Buch M, Emery P. The aetiology and pathogenesis of rheumatoid arthritis. Hosp Pharm. 2002;9:5-10.
2. Sierakowski S, Cutolo M. Morning symptoms in rheumatoid arthritis: a defining characteristic and marker of active disease. Scand J Rheumatol. 2011;40(suppl 1):251-255.
3. National Collaborating Centre for Chronic Conditions. Rheumatoid Arthritis: National Clinical Guideline for Management and Treatment in Adults. London, UK: Royal College of Physicians; February 2009.
4. Smolen JS, Aletaha D, Koeller M, et al. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861-1874.
5. Myasoedova E, Davis JM, Crowson CS, Gabriel SE. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12:379-385.
6. Gonzalez A, Maradit KH, Crowson CS, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56:3583-3587.
7. Clarke A, Vyse TJ. Genetics of rheumatic disease. Arthritis Res Ther. 2009;11:248.
8. Carmona L, Cross M, Williams B, et al. Rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2010;24:733-745.
9. Ramsburg K. Rheumatoid arthritis. Am J Nurs. 2000;100:40-43.
10. Sweetman SC, ed. Martindale: The Complete Drug Reference. 34th ed. London, UK: Pharmaceutical Press; 2005 [electronic version].
11. Dougados M, Smolen JS. Pharmacological management of early rheumatoid arthritis—does combination therapy improve outcomes. J Rheumatol Suppl. 2002;66:20-26.
12. Sokka T, Mäkinen H. Drug management of early rheumatoid arthritis—2008. Best Pract Res Clin Rheumatol. 2009;23:93-102.
13. Graudal N, Jürgens G. Similar effects of disease-modifying antirheumatic drugs, glucocorticoids, and biologic agents on radiographic progression in rheumatoid arthritis: meta-analysis of 70 randomized placebo-controlled or drug-controlled studies, including 112 comparisons. Arthritis Rheum. 2010;62:2852-2863.
14. American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis—2002 update. Arthritis Rheum. 2002;46:328-346.
15. Carteron N. Cytokines in rheumatoid arthritis: trials and tribulations. Mol Med Today. 2000;6:315-323.
16. Tak PP, Kalden JR. Advances in rheumatology: new targeted therapeutics. Arthritis Res Ther. 2011;13(suppl 1):S5.
17. Rosenbaum CC, O’Mathúna DP, Chavez M, Shields K. Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern Ther Health Med. 2010;16:32-40.
To comment on this article, contact rdavidson@uspharmacist.com.






