US Pharm. 2006;7:42-48.
Exercise-induced
asthma (EIA) is a condition characterized by airway obstruction following
exercise. Symptoms include wheezing, shortness of breath, chest tightness or
pain during or after exercise, coughing, and difficulty breathing. Some
patients may have more subtle symptoms not clearly recognized as being due to
asthma, such as cramps, stomach pain, sore throat, and headache. EIA is common
in patients with chronic asthma, but it is also seen in those who do not have
other forms of asthma. In athletes, these symptoms may be misinterpreted as a
prolonged recovery time or being "out of shape." EIA may affect people of any
age and at any level of exercise.1,2
Exercise-induced bronchospasm
(EIB) is an intermittent bronchial narrowing occurring after exercise in those
with normal lung function at rest. The main difference between EIB and EIA is
the absence of subjective symptoms in EIB, despite a measurable drop in
airflow.1,2 Chronic asthma is an inflammatory disorder that results
in airway hyperreactivity (AHR) to various stimuli. Cold air inhalation, mold,
pollen, animal dander, upper respiratory infections, and exercise are common
triggers for AHR in patients with chronic asthma. In 45% to 90% of these
patients, exercise can significantly decrease the forced expiratory volume in
one second (FEV1).3 Inflammation and AHR cause recurrent
episodes of chest tightness, wheezing, breathlessness, and coughing. If the
inflammation goes unchecked, airway remodeling may occur, possibly leading to
chronic irreversible airway obstruction.1,3
Some individuals with EIA have
symptoms only with exercise and do not otherwise have asthma. This may be a
normal physiologic response or may be due to the extreme exercise of top
athletes, e.g., athletes competing in the Olympics.4 EIA can occur
in up to 50% of cold-weather athletes, possibly a direct result of inhalation
of large volumes of cold air. Studies report airway inflammation in
cross-country skiers and speed skaters, but these subjects did not respond to
inhaled steroids or short-acting beta-2 agonists, suggesting a condition
unique to cold-weather athletes that differs from asthma.5,6
Etiology
EIA's underlying
mechanisms are not fully understood; however, two hypotheses have been
proposed:the water-loss and postexercise rewarming hypotheses. The first
theorizes that evaporation from respiratory mucosa during exercise results in
hyperosmolarity within the airway cells. This leads to mast cell release of
inflammatory mediators, e.g., histamine, prostaglandins, chemotactic factors,
and leukotrienes, causing vasodilation and bronchial smooth muscle
contraction, which eventually results in airway obstruction. The obstruction
may ultimately worsen the condition because inflammation may facilitate
further water loss.1,2,7
The postexercise rewarming
hypothesis suggests that hyperventilation from exercise causes heat loss from
the respiratory mucosa, reducing bronchial blood flow. After exercise, a
rewarming process causes dilatation of the bronchiolar vessels around the
airways, leading to reactive hyperemia of the airway lining, vascular
engorgement, edema, and subsequent mediator release and airway obstruction.
1,2,7
Prevalence
At least 11% to 15%
of children, adolescents, and adults are estimated to have EIA.2 It
occurs in up to 90% of those with chronic asthma.8 In the 1996
Summer and 1998 Winter Olympics, about 17% of athletes reported a previous
case of asthma, with the highest occurrence in endurance athletes.9-11
In the 2002 Olympics, about 15% of cross-country skiers used beta-agonists
for EIA.12 The highest incidence is found in competitive athletes
in cold-weather sports, with an overall incidence of 23%; in cross-country
skiers, the incidence is as high as 50%.10
Diagnosis
EIA is often
underdiagnosed due to an individual's denial of symptoms. This denial is
commonly because of peer pressure, embarrassment, fear of losing one's
position in team sports, or the misinterpretation of postexercise fatigue. The
first step in diagnosis is to rule out chronic asthma. Certain factors in the
patient's history help increase the chance of an EIA diagnosis. These include
increased symptoms with continuous hard exercise, such as running; exercise in
a cold environment, in polluted air, during the pollen season, or during a
respiratory infection; a family history of asthma; or a personal history of
recurrent allergic rhinitis or sinsusitis.1,3 To confirm the
diagnosis of EIA, a standardized exercise challenge that includes spirometry
is recommended. This involves a treadmill, cycle, or free-running asthma
screening test to induce symptoms and measurement of FEV1.2
A drop of at least 10% in FEV1 is typically required for diagnosis;
2 however, a 20% to 25% drop in FEV1 has been suggested.
13 Another test is the eucapnic voluntary hyperventilation challenge
with dry air, recommended by the International Olympic Committee (IOC) Medical
Commission for testing of Olympic athletes with asthma. Disadvantages with
this challenge are the expensive equipment required and the complexity of the
test.1
Nonpharmacologic Management
Management
initially involves discussion with the individual on the types of exercise
least likely to induce EIA: intermittent exercise or team sports; swimming;
and exercise in nonpolluted air, outside of the pollen season, or in warm,
humid air.1,2 Athletes should also be encouraged to follow a
regular regimen of warm-up and cool-down to minimize symptoms. A warm-up to
about 80% of maximal output before a full routine has been shown to partially
reduce the severity of EIA. A gradual cool-down is beneficial to minimize
postexercise fatigue.14
Pharmacologic Management
Eight main
medication classes are used in the pharmacologic management of EIA:
short-acting beta-agonists, long-acting beta-agonists, mast cell stabilizers,
inhaled corticosteroids, anticholinergics, leukotriene antagonists,
antihistamines, and anti-IgE monoclonal antibodies (Table 1). Therapy
should be individualized based on severity of symptoms and level of exercise
performed.
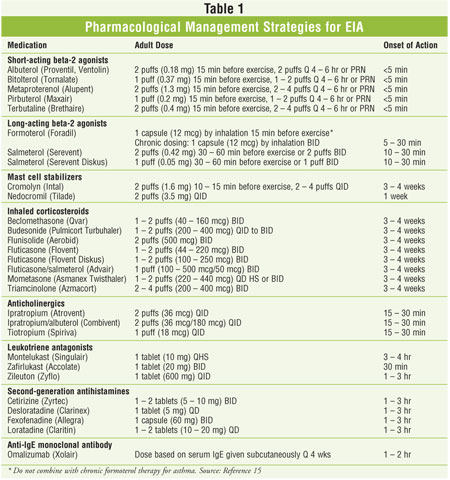
Both the short-acting and
long-acting beta-agonists are direct-acting sympathomimetic agents with
selective activity on beta-2 adrenoceptors, with the long-acting agents
displaying greater selectivity for these receptors than the short-acting
agents. These agonists cause bronchial smooth muscle relaxation and inhibit
release of immediate hypersensitivity mediators from mast cells.15
First-line therapy is a short-acting beta-agonist administered five to 15
minutes prior to exercise.3 Side effects are typically minor but
may include palpitations, tremor, or tachycardia. A long-acting beta-agonist
(e.g., salmet erol) may be given several hours before exercise; however, a
short-acting beta-agonist must also be provided for acute relief of symptoms.
As treatment for EIA, long-acting beta-agonists are most effective when used
intermittently and when the short-acting beta-agonists are not used on a daily
basis. Regular daily use may result in tolerance to the product due to
down-regulation of the beta-2 receptors on the airway cells.1-3
A new black box warning has
recently been added to the prescribing information for long-acting beta-2
agonists. Data from a recent placebo-controlled trial showed an increase in
asthma-related deaths in patients receiving salmeterol, compared to those on
placebo (0.099% vs. 0.023%, respectively).These agents should be prescribed
only to patients whose asthma symptoms are not controlled on other asthma
medications or whose disease severity warrants the addition of these agents.
If EIA is not controlled with
a short-acting beta-agonist, the mast cell stabilizers--cromolyn or
nedocromil--may be added to the regimen. They inhibit the release of several
mediators of inflammation from various cell types, e.g., mast cells,
macrophages, eosinophils, neutrophils, monocytes, and platelets.15
Their efficacy lasts for only about two hours, and they do not cause
bronchodilation. Side effects reported are bad taste, headache, dizziness,
nausea, sore throat, and stinging eyes.1-3
The leukotriene antagonists
such as montelukast have been efficacious to treat EIA when used as adjunctive
therapy. These agents selectively bind to the cysteinyl leukotriene 1
receptors found in airway smooth muscle and airway macrophages, preventing the
binding of cysteinyl leukotrienes to these receptors. Leukotriene-mediated
effects in asthma include smooth muscle contraction, airway edema, and
inflammatory reactions.15 Leff et al. reported significant
protection against EIB with montelukast vs. placebo.16 Again, a
short-acting beta-agonist must be provided for acute relief of symptoms. All
three drugs in this class are metabolized by the cytochrome P450 (CYP) system.
Zileuton is metabolized by CYP 1A2, 2C9, and 3A4; montelukast by CYP 3A4, 2C9,
and 2A6; and zafirlukast by CYP 2C9. Therefore, significant drug interactions
are possible. Adverse events are usually mild and include headache,
somnolence, and nausea. A Churg––Strauss-like syndrome (CSS), a rare and
potentially fatal reaction, has been reported as a complication in asthmatics
who are steroid-dependent and are treated with a leukotriene antagonist. This
syndrome typically occurs in association with reduction of their oral steroid
dose. CSSis a granulomatous vasculitis that affects small- to medium-sized
vessels.1-3
The anticholinergics
antagonize the action of acetylcholine, blocking the bronchoconstriction
caused by acetylcholine and methacholine.15 Both ipratropium and
tiotropium have shown efficacy in EIA, with peak bronchial effects attained at
one to two hours. Due to a duration of action of only three to six hours,
these drugs are often dosed three to four times daily. Their most frequent
adverse effects are dry mouth and cough.1-3
Inhaled corticosteroids are
typically reserved for those with EIA who also have chronic asthma. They are
often given in combination with a long-acting beta-agonist.1-3 They
have potent glucocorticoid but weak mineralocorticoid activity, together with
potent anti-inflammatory effects.15 Adverse effects are generally
mild and include pharyngitis and oropharyngeal candidiasis if the mouth is not
rinsed after use. Pretreatment before exercise is advised, and a short-acting
beta-agonist should be available to control acute symptoms.1-3
Second-generation
antihistamines can be used for patients with both EIA and atopy. Atopy is an
inherited type I hypersensitivity or allergic reaction involving elevated
immunoglobulin E (IgE), resulting in hay fever, asthma, or such skin problems
as urticaria or eczema. First-generation antihistamines are not typically used
in asthma because of their anticholinergic effects, which can result in
the drying of bronchial secretions. In contrast, second-generation
antihistamines have greater selectivity for the H1receptor and fewer
anticholinergic side effects, and they may inhibit other inflammatory
processes involved in asthma. Adverse effects are typically mild, e.g.,
nausea, headache, drowsiness, and dry mouth.15
Omalizumab (Xolair) may be an
alternative for those with EIA and IgE-mediated chronic asthma. Omal izumab
is an anti-IgE monoclonal antibody given by subcutaneous injection. Its major
drawback is its cost, ranging from $600 to $700 per month.17 It is
generally well tolerated; urticarial rashes and injection site reactions are
the most common adverse reactions.15
Professional athletes should
be advised to check with the appropriate athletic governing bodies regarding
which medications are permitted for a given professional competition. Both the
IOC and the National Collegiate Athletic Association established guidelines
for allowable medications to prevent the use of performance-enhancing agents
during competitive sports.
Dietary Supplements
Accumulating evidence suggests that
a diet low in salt and high in omega-3 fatty acids and antioxidants can reduce
the incidence of EIA. Animal studies indicate that salt loads can affect
leukotriene release. Many studies show a beneficial effect of a low-salt diet
of about 1,500 mg per day, while other authors suggest less than 2,400 mg per
day of sodium to reduce the severity of EIA.8,18,19
Eicosapentaenoic and docosahexaenoic acid are omega-3 polyunsaturated fatty
acids found in fish oils. These agents competitively inhibit arachidonic acid
metabolism, thereby reducing the generation of inflammatory prostaglandins and
leukotriene mediators, as well as the inflammatory cell production of
cytokines. Therefore, it has been postulated that diets high in fish oils may
reduce diseases caused by inflammation, including EIA. To date, clinical data
of the short-term use of fish oil supplements for asthma are controversial.
Further clinical trials are needed to evaluate the effects of omega-3 fatty
acids in people with asthma.17,20,21
Evidence suggests that
oxidants produced during the inflammatory process may contribute to asthma;
thus, antioxidants may be effective in reducing the severity of EIA. Ascorbic
acid (vitamin C), in doses ranging from 500 to 2,000 mg taken one to two hours
before exercise, has been shown to improve EIA to subclinical levels in
several clinical trials.22-24 Beta-carotene, dosed at 64 mg daily
for one week, and lycopene, dosed at 30 mg daily for one week, have also
demonstrated efficacy.25,26
Caffeine causes bronchiolar
smooth muscle relaxation and can reduce EIA severity. However, the doses
required to achieve this exceed the limits permitted for international
competition (<12 mcg/mL in urine) and are likely to result in disqualification
from professional athletic events. The doses of caffeine required to show
benefit (7 to 10 mg/kg taken 90 minutes to two hours prior to exercise) are
also likely to cause significant diuresis.27,28
Conclusions
Proper treatment of EIA is essential
to enable those affected to participate in sports and activities from which
they might otherwise be restricted. Management should focus on prevention and
the nonpharmacologic and pharmacologic therapy specifically tailored to the
individual.
References
1. Storms WW.
Review of exercise-induced asthma. Med Sci Sports Exerc.
2003;35:1464-1470.
2. Weiler JM.
Exercise-induced asthma: a practical guide to definitions, diagnosis,
prevalence, and treatment. Allergy Asthma Proc. 1996;17:315-325.
3. National Asthma
Education and Prevention Program. Expert panel report: guidelines for the
diagnosis and management of asthma update on selected topics--2002. J Allergy
Clin Immunol. 2002;110(5 suppl):S141-S219.
4. Anderson SD, Holzer
K. Exercise-induced asthma: is it the right diagnosis in elite athletes? J
Allergy Clin Immunol. 2000;106:419-428.
5. Sue-Chu M,
Karjalainen EM, Laitinen A, et al. Placebo-controlled study of inhaled
budesonide on indices of airway inflammation in bronchoalveolar lavage fluid
and bronchial biopsies in cross-country skiers. Respiration. 2000;67:417-425.
6. Wilber RL, Rundell
KW, Judelson DA. Efficacy of asthma medication regimen in elite athletes with
exercise-induced asthma. Med Sci Sports Exerc. 2001;33:S12.
7. Cypcar D, Lemanske
RF Jr. Asthma and exercise. Clin Chest Med. 1994;15:351-368.
8. Mickleborough TD,
Gotshall RW. Dietary salt intake as a potential modifier of airway
responsiveness in bronchial asthma. J Altern Complement Med. 2004;10:633-642.
9. Weiler J, Ryan EJ.
Asthma in United States Olympic athletes who participated in the 1998 Olympic
winter games. J Allergy Clin Immunol. 2000;106:267-271.
10. Wilber RL, Rundell
KW, Szmedra L, et al. Incidence of exercise-induced bronchospasm in Olympic
winter sport athletes. Med Sci Sports Exerc. 2000;32:732-737.
11. Weiler JM, et al.
Asthma in United States Olympic athletes who participated in the 1996 Summer
Games. J Allergy Clin Immunol. 1998;102:722-726.
12. Anderson SDK, Fitch
K, Perry CP, et al. Responses to bronchial challenge submitted for approval to
use inhaled beta2-agonists before an event at the 2002 Winter Olympics. J
Allergy Clin Immunol. 2003;111:45-50.
13. Tan RA, Spector SL.
Exercise-induced asthma. Sports Med. 1998;25:1-6.
14. McKenzie DC,
McLuckie SL, Stirling DR. The protective effects of continuous and interval
exercise in athletes with exercise-induced asthma. Med Sci Sports Exerc.
1994;26:951-956.
15. Wickersham RM, ed.
Drug Facts and Comparisons. St. Louis, MO: Facts and Comparisons; 2006.
16. Leff JA, Busse WW,
Pearlman D, et al. Montelukast, a leukotriene-receptor antagonist, for the
treatment of mild asthma and exercise-induced bronchoconstriction. N Engl J
Med. 1998;339:147-152.
17. Fleming T, editor.
2006 Drug Topics Red Book. Pharmacy's Fundamental Reference. Montvale, NJ:
Medical Economics Co.; 2006.
18. Mickleborough TD,
Gotshall RW. Dietary components with demonstrated effectiveness in decreasing
the severity of exercise-induced asthma. Sports Med. 2003;33:671-681.
19. Mickleborough TD,
Gotshall RW, et al. Dietary salt alters pulmonary function during exercise in
exercise-induced asthmatics. J Sports Sci. 2001;19:865-873.
20. Stephensen CB. Fish
oil and inflammatory disease: is asthma the next target for n-3 fatty acid
supplements? Nutr Rev. 2004;62:486-489.
21. Mickleborough TD,
Murray RL, Lindley MR. Elevating dietary omega-fatty acid consumption reduces
the severity of exercise-induce bronchoconstriction. Med Sci Sports Exerc
. 2003;35:S10.
22. Hatch GE. Asthma,
inhaled oxidants, and dietary antioxidants. Am J Clin Nutr. 1995;61(3
suppl):625S-630S.
23. Schachter EN,
Schlesinger A. The attenuation of exercise-induced bronchospasm by ascorbic
acid. Ann Allergy. 1982;49:146-151.
24. Cohen HA, Neuman I,
Nahum H. Blocking effect of vitamin C in exercise-induced asthma. Arch
Pediatr Adolesc Med. 1997;151:367-370.
25. Neuman I, et al.
Prevention of exercise-induced asthma by a natural isomer mixture of
beta-carotene. Ann Allergy Asthma Immunol. 1999;82:549-553.
26. Neuman I, Nahum H,
Ben-Amotz A. Reduction of exercise-induced asthma oxidative stress by
lycopene, a natural antioxidant. Allergy. 2000;55:1184-1189.
27. Kivity S, Ben
Aharon Y, Man A, et al. The effect of caffeine on exercise-induced
bronchoconstriction. Chest. 1990;97:1083-1085.
28. Duffy P, Phillips
YY. Caffeine consumption decreases the response to bronchoprovocation
challenge with dry gas hyperventilation. Chest. 1991;99:1374-1377.
To comment on this article, contact
editor@uspharmacist.com.






