US Pharm. 2021;46(11):31-42.
ABSTRACT: Obesity is one of the leading causes of type 2 diabetes, and it has continued to rise in incidence and prevalence in recent years. Many diabetes-related factors, such as diabetes medications and alterations in glucose metabolism, contribute to an increased risk of obesity. A core element to selecting glucose-lowering therapies, according to current diabetes guidelines, is the medication’s impact on weight. Classes of medications such as metformin, glucagon-like peptide-1 agonists, and sodium-glucose cotransporter 2 inhibitors aid in weight loss and improve overall cardiovascular health. Pharmacists should review treatment plans and counsel patients about incorporating diet and lifestyle changes for optimal outcomes.
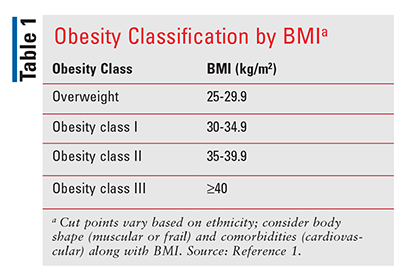
Obesity is one of the predominant risk factors in the development of type 2 diabetes (T2D). The definition of obesity, based on the American Diabetes Association (ADA) guidelines, is subdivided into classes determined by BMI (TABLE 1).1 The development of T2D as an obesity-related condition is among the leading causes of preventable, premature death.2 According to the CDC, among U.S. adults aged 18 years or older with diagnosed diabetes from 2013 to 2016, 89.0% were overweight or obese, defined as a BMI of 25 kg/m2 or higher.3 In addition, the CDC has found that the rise in the number of diabetes cases in the past decade has been largely influenced by the rise in obesity in the U.S., as rates of obesity increased from 30.5% to 42.4% from 2000 to 2018, and the prevalence of severe obesity rose from 4.7% to 9.2%.2 The relationship between diabetes and obesity is a two-way street: Obesity leads to a heightened risk of diabetes, and the presence of diabetes in patients with obesity contributes to disease progression and cardiovascular complications.4 In general, a higher BMI increases the risk of diabetes, cardiovascular disease, lower quality of life, and all-cause mortality.1
Owing to the awareness of these complications and important interplay between obesity and diabetes, there has been a recent shift in the development of both glucose-lowering therapies and specific guidelines for managing obesity in patients with T2D in order to improve outcomes and glycemic control. Patients with T2D obtain substantial benefits with even moderate weight loss; as little as 2% to 3% weight loss from baseline can reduce the A1C by 0.2% to 0.3%.5 Even more profound weight-loss achievements in the 5% to 15% range from baseline can yield a 0.5% to 1% reduction in A1C, thereby reducing the need for pharmacotherapy while also improving cardiovascular parameters.6 Given the benefits provided by obesity management, diabetes treatment should follow a multimodal approach, with weight loss and/or management as its core element for improving overall patient outcomes.
Risk Factors for Obesity
A major limitation in the historical treatment of diabetes is that the conventional glucose-lowering therapies contribute to significant amounts of weight gain. The most predominant glucose-lowering therapy that may effect weight gain is insulin. By decreasing glycosuria, increasing glucose uptake into adipose tissue, and inducing hypoglycemia (resulting in higher calorie intake), insulin’s contribution to weight gain is multifactorial.7 Unfortunately, insulin remains a cornerstone therapy for patients with uncontrolled or prolonged disease. In addition, one of the oldest drug classes for diabetes treatment, sulfonylureas, contributes to substantial weight gain of 0.6 to 1.8 kg, depending on the type of insulin secretagogues (lower risk with meglitinides) and length of treatment.7 However, insulin secretagogues remain a convenient and important low-cost, oral option that is widely used in clinical practice. The use of thiazolidinediones is also associated with weight gain, as these agents have been shown to render adipose tissue more insulin sensitive.7 In the PROactive study, weight gain of 3.8 kg was observed with pioglitazone over a 3-year period.8 Concurrent therapies, such as antipsychotics or antiepileptic drugs used to treat comorbidities and/or diabetic peripheral neuropathy, add to the risk of weight gain.1 These factors and more perpetuate the issue of continued weight gain and make the process more difficult for patients with diabetes to achieve their desired weight-loss goals.
On the other hand, therapies with weight-neutral or even weight-loss properties can improve glycemic control as well as the chances of attaining an A1C goal of 7% or lower.9 These therapies include glucagon-like peptide-1 (GLP-1) receptor agonists, sodium-glucose cotransporter 2 (SGLT2) inhibitors, and biguanides, which promote weight loss, as well as dipeptidyl peptidase-4 inhibitors, alpha-glucosidase inhibitors, and bile-acid sequestrants, which are weight neutral.5
Goals of Therapy
According to the ADA guidelines, there is a strong and consistent evidence base to support that vigilant obesity management can delay the progression from prediabetes to T2D and improve glycemic control as well as reduce the need for glucose-lowering therapies in patients with established T2D.1,5 Therefore, weight management forms the core of the treatment plan in patients with diabetes. However, weight reduction remains a significant challenge owing to multiple factors such as glucose-lowering therapies, insufficient lifestyle modifications, and socioeconomic status. Before deciding on a goal for a given patient, it is critical to be aware of the patient’s perspective on his or her weight, cultural or economic barriers, and correct BMI classification. BMI cut points may differ based on ethnicity (e.g., Asian and Asian American populations define obesity at a lower cut point owing to differences and cardiovascular risk) as well as on whether the individual is highly muscular or is frail.1,5
The goal of therapy for weight loss is stepwise, successive, and individualized. Generally, clinical benefits of weight loss manifest after a weight loss of 3% to 5% is achieved, with greater overall benefits observed in those who attain a weight loss above this threshold.1 In terms of lifestyle interventions, the ADA guidelines provide recommendations for dietary intervention and exercise patterns that promote a successful weight-loss plan. A caloric deficit of 500 to 750 kcal/day is recommended, with the differences in the daily caloric goal based on gender and baseline body weight. In addition, exercise and behavioral strategies, as determined in consultation with a trained interventionist, are strongly recommended.1 Also, a patient’s medication list should undergo a thorough review in order to facilitate discussion about potentially changing or discontinuing any concomitant medications that promote weight gain (e.g., steroids, antipsychotics, antidepressants, progestins, anticonvulsants, and antihistamines).1
Pharmacologic Therapy
Medications used for the management of T2D have variable effects on weight; some demonstrate favorable weight-loss effects, some have weight neutrality, and others cause weight gain. When weight loss is desired, a review of the current medication list is essential in order to assess for potential areas for optimization in terms of blood-glucose control and weight management. Medications that have demonstrated a favorable effect on weight loss, including metformin, SGLT2 inhibitors, and GLP-1 agonists, will be discussed in detail, including their impact on weight loss in patients with T2D (TABLE 2).

Metformin
Metformin remains first-line therapy for blood-glucose control in patients with T2D and has demonstrated beneficial effects on weight management, including weight loss or weight neutrality.1 The mechanism underlying the weight effect of metformin is proposed to be secondary to suppression of hepatic gluconeogenesis, improvement in peripheral insulin sensitivity, and reduction in intestinal glucose absorption. Metformin has also been shown to have a dose-dependent impact on leptin levels and induce appetite suppression.10 The weight loss associated with metformin treatment is preferentially loss of adipose tissue and visceral fat.11
The degree of weight loss with metformin has varied in the literature (0.6%-13%) given the frequent use of diabetic medications known to influence body weight (e.g., insulin, sulfonylureas) and varied adherence to lifestyle modifications.1,12 In a randomized, controlled trial, women with obesity and non–insulin-dependent diabetes were randomized to treatment with metformin 850 mg twice daily or placebo as an adjunct to caloric restriction to assess the impact on weight loss. At 24-week follow-up, women treated with metformin had an 8-kg greater weight loss compared with placebo (P <.001).10 The investigators proposed that the significant impact on weight loss was secondary to reduced calorie consumption and increased satiety in the metformin cohort, an effect that was dose dependent.10
Metformin has also been investigated in the treatment of prediabetes as a part of the Diabetes Prevention Program.13 Patients with prediabetes treated with metformin lost 2.5% of their body weight.13 A randomized, controlled trial of metformin versus placebo in insulin-resistant children aged 6 to 12 years demonstrated superiority of metformin in body weight, body composition, and glucose homeostasis.14 Use of metformin for weight reduction in these populations may result in favorable long-term outcomes in preventing complications of obesity such as T2D, cardiovascular disease, and other obesity-related comorbid conditions.
Metformin is associated with dose-limiting gastrointestinal-related adverse effects. Nausea, vomiting, and diarrhea occur in 20% to 30% of patients; therefore, slow dose titrations are necessary to mitigate these effects.15 Tolerability can further be improved by counseling the patient to take metformin with a meal and/or transitioning the patient to the extended-release tablet formulation. Metformin is renally cleared and should be avoided in patients with an estimated glomerular filtration rate <30 mL/min, given the heightened risk of lactic acidosis in this population.15 Nutritional counseling for patients who take metformin is essential, as vitamin B12 deficiency has been associated with long-term use, and supplementation is recommended.16
GLP-1 Agonists
GLP-1, an incretin hormone produced in the gastrointestinal tract, is primarily responsible for regulating appetite and insulin secretion in a glucose-dependent manner.17 GLP-1 agonists are recommended as adjuncts to metformin treatment in T2D when promoting weight loss, minimizing weight gain, or reducing cardiovascular risk is desired.15 Five GLP-1 agonists have been FDA approved for management of T2D; these include dulaglutide, exenatide, liraglutide, lixisenatide, and semaglutide, all of which have demonstrated beneficial effects on weight loss.15 The mechanisms by which these agents promote weight loss include reduced hepatic gluconeogenesis and decreased gastric emptying/gut motility, in addition to a role in the promotion of satiety.17
Weight loss with GLP-1 agonists is a class effect, with an estimated 3-kg to 10-kg reduction depending on the agent used.1 A 28-week trial that examined the addition of exenatide or glargine to already-prescribed oral agents found an equal decrease in A1C, with weight gain in the insulin cohort of 1.2 kg and weight loss in the exenatide cohort of 2.0 kg.18 After 30 weeks, exenatide caused a reduction in weight from baseline of 3.0 kg, with a progressive reduction in weight of 5.3 kg after 82 weeks.18 In a trial evaluating the impact of liraglutide on weight loss in T2D and obesity, an average weight loss of 6.4 kg was seen with liraglutide 3 mg daily and 5-kg loss with liraglutide 1.8 mg daily, versus 2.2 kg with placebo, at 56-week follow-up.19 In a 68-week follow-up study, semaglutide 2.4 mg demonstrated a 9.7-kg weight loss versus 3.5 kg with lifestyle interventions alone.20 Post hoc analysis of the SUSTAIN trials comparing semaglutide with alternative GLP-1 agonists suggests superiority of semaglutide for weight loss and blood-glucose control, an effect that was independent of the presence of gastrointestinal adverse events.21 Notably, the effect of GLP-1 agonists on weight is dose dependent; therefore, patients should be titrated to the maximum dose in order to sustain maximum benefits, if tolerated.
Important considerations when selecting the optimal GLP-1 agonist include agent and formulation for optimizing weight-loss strategies. Exenatide is available as a twice-daily (Byetta) or once-weekly (Bydureon) formulation. The once-weekly formulation has consistently demonstrated improved weight-loss properties compared with the twice-daily formulation.22 Liraglutide is also commercially available as a 3-mg injection (Saxenda) that is specifically indicated for weight-loss management. It is essential to avoid incorrect prescribing of this agent and the 1.8-mg injection (Victoza) indicated for diabetes management. Lixisenatide is formulated as an injectable alone (Adlyxin) or combined with insulin glargine (Soliqua). The combination of GLP-1 and insulin may help mitigate the weight gain from insulin monotherapy; however, consideration of therapies that mitigate weight gain overall would be preferable, if appropriate. For patients in whom SC injections are not preferable, the oral GLP-1 agonist semaglutide is a safe and effective alternative that demonstrated similar effects on weight control. The most significant adverse events from GLP-1 agonists include gastrointestinal side effects such as nausea and vomiting secondary to impaired gastric emptying. This effect may be exacerbated in patients with preexisting diabetic gastroparesis. Slow dose titration and once-weekly dosing formulations can help mitigate gastrointestinal-related adverse events.
SGLT2 Inhibitors
SGLT2 is located in the proximal tubule of the kidney and is responsible for about 80% to 90% of blood-glucose reabsorption.23 SGLT2 inhibitors, including dapagliflozin, canagliflozin, ertugliflozin, and empagliflozin, reduce plasma glucose concentrations through glucose inhibition and sodium reabsorption from the kidneys, resulting in glucosuria. Weight loss with SGLT2 inhibitors is mediated by glycosuria at an estimated 75 g/day inducing a 300-calorie deficit.23 Additionally, inhibition of sodium and glucose reabsorption can result in an osmotic diuresis, further promoting weight reduction through minimizing water retention.23 Unlike alternative antidiabetics, SGLT2 inhibitors offer an insulin-independent mechanism for improving blood glucose and promoting weight control.
Monotherapy with SGLT2 inhibitors as a class results in a weight loss of approximately 2 kg.1,23 This weight-loss effect is dose dependent.24 When a SGLT2 inhibitor is added to metformin or GLP-1 agonists, an additional 3-kg to 5-kg weight loss can be seen.1,23 Weight loss is maintained with long-term treatment, as shown by a 2-year follow-up study of dapagliflozin treatment that demonstrated sustained fat-mass reduction over the study period.25 Although the effect on weight loss may be modest, SGLT2 inhibitors have been demonstrated to have a positive impact on blood pressure and reduction of major cardiovascular and renal events that may have significant implications for reducing cardiovascular morbidity in this population.26 Evidence suggests that the beneficial effects of SGLT2 inhibitors on weight may be offset by a compensatory increase in caloric intake. Therefore, employing a multimodal approach and combining SGLT2 inhibitors with agents that work via different mechanisms (e.g., GLP-1 agonists and metformin), in addition to dietary and lifestyle modification, are imperative to ensuring that the patient’s weight loss is maintained.
The Pharmacist’s Role
Pharmacists play an essential role in optimizing treatment plans for patients with diabetes and comorbid obesity. A comprehensive review of a patient’s medications, including antidiabetic and non-antidiabetic medications, should be performed to assess potential agents that can contribute to weight gain (e.g., antipsychotics, insulin, corticosteroids). Given that the effect of most antidiabetic medications on weight loss is dose dependent, every effort should be made to ensure that the patient is titrated appropriately to the adequate doses to maximize treatment benefits. A counseling session should be performed to make sure that the patient is well informed about the potential adverse events associated with these medications. Gastrointestinal intolerances are common, and patients should be instructed on how to mitigate these events (e.g., take metformin with a meal) to improve tolerability of treatment. Close monitoring and follow-up are critical to optimize treatments, and any signs or symptoms of adverse events should be reported. Pharmacists should emphasize that medications are most effective when used as adjuncts to diet and lifestyle modifications.1 Therefore, educational efforts directed toward healthy dietary habits and physical-activity plans are essential to optimizing patient outcomes. If medication and lifestyle modifications are insufficient to support the patient’s weight-loss goals, the pharmacist should consider referring the patient for metabolic surgery, if the necessary criteria are met.
REFERENCES
1. American Diabetes Association. 8. Obesity management for the treatment of type 2 diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S100-S110.
2. CDC. Overweight & obesity: obesity is a common, serious, and costly disease. www.cdc.gov/obesity/data/adult.html. Accessed October 7, 2021.
3. CDC. Diabetes: risk factors for diabetes-related complications. www.cdc.gov/diabetes/data/statistics-report/risks-complications.html. Accessed October 7, 2021.
4. Van Gaal L, Scheen A. Weight management in type 2 diabetes: current and emerging approaches to treatment. Diabetes Care. 2015;38(6):1161-1172.
5. Kahan S, Fujioka K. Obesity pharmacotherapy in patients with type 2 diabetes. Diabetes Spectr. 2017;30(4):250-257.
6. Wing RR, Lang W, Wadden TA, et al; Look AHEAD Research Group. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481-1486.
7. Provilus A, Abdallah M, McFarlane SI. Weight gain associated with antidiabetic medications. Therapy. 2011;8(2):113-120.
8. Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial in macroVascular events): a randomised controlled trial. Lancet. 2005;366(9493):1279-1289.
9. McAdam-Marx C, Mukherjee J, Bellows BK, et al. Evaluation of the relationship between weight change and glycemic control after initiation of antidiabetic therapy in patients with type 2 diabetes using electronic medical record data. Diabetes Res Clin Pract. 2014;103(3):402-411.
10. Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res. 1998;6(1):47-53.
11. Stumvoll M, Nurjhan N, Perriello G, et al. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333(9):550-554.
12. Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):323-329.
13. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403.
14. Yanovski JA, Krakoff J, Salaita CG, et al. Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial. Diabetes. 2011;60(2):477-485.
15. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S111-S124.
16. Aroda VR, Edelstein SL, Goldberg RB, et al. Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab. 2016;101(4):1754-1761.
17. Shah M, Vella A. Effects of GLP-1 on appetite and weight. Rev Endocr Metab Disord. 2014;15(3):181-187.18. Heine RJ, Van Gaal LF, Johns D, et al; GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143(8):559-569.19. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687-699.
20. Davies M, Faerch L, Jeppesen OK, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971-984.
21. Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diab Res Care. 2020;8(2):e001706.
22. De Block CE, Van Gaal LF. Efficacy and safety of exenatide once weekly: an overview of the DURATION trials. Expert Rev Endocrinol Metab. 2012;7(6):611-623.
23. Pereira MJ, Eriksson JW. Emerging role of SGLT-2 inhibitors for the treatment of obesity. Drugs. 2019;79(3):219-230.
24. Cai X, Yang W, Gao X, et al. The association between the dosage of SGLT2 inhibitor and weight reduction in type 2 diabetes patients: a meta-analysis. Obesity (Silver Spring). 2018;26(1):70-80.
25. Bolinder J, Ljunggren O, Johansson L, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2014;16(2):159-169.
26. Rabizadeh S, Nakhjavani M, Esteghamati A. Cardiovascular and renal benefits of SGLT2 inhibitors: a narrative review. Int J Endocrinol Metab. 2019;17(2):e84353.
27. Saxenda (liraglutide) package insert. Plainsboro, NJ: Novo Nordisk Inc; December 2020.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





