US Pharm. 2013;38(5):38-41.
ABSTRACT: Infectious mononucleosis (IM) is a viral infection with symptoms consisting of fever, lymphadenopathy, pharyngitis, and fatigue. Most cases of symptomatic IM occur between the ages of 15 and 24 years. IM is generally a self-limiting disease with symptoms resolving in a few weeks. Pharmacologic therapy generally consists of supportive care with nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen. Pharmacists can play a key role in patient outcomes by recommending supportive care measures and counseling on the need to limit strenuous activities and become aware of accompanying fatigue.
Infectious mononucleosis (IM), otherwise known as mono, the kissing disease, or glandular fever, is an infectious viral disease caused by the Epstein-Barr virus (EBV).1 EBV is a ubiquitous herpes virus that is found in all human societies and cultures.2
Epidemiology
Over 95% of adults worldwide have been infected with EBV.1 EBV infection is rare during the first year of life, which could be due to passive immunity from mother to child. The age of initial infection varies depending upon cultural and socioeconomic upbringing. The earlier in life EBV infection occurs, the milder the symptoms are. Infection occurring in early childhood is often asymptomatic or causes such insignificant illness that it is rarely ever identified as EBV. In contrast, EBV infection occurring in adolescence or adulthood produces symptomatic IM illness.1-3
In developing countries or lower socioeconomic classes, 80% to 100% of children become seropositive for EBV by 6 years of age, and therefore largely do not express symptomatic IM illness. In higher socioeconomic groups or developed countries, only 50% of children become seropositive for EBV between the ages of 1 and 5 years; therefore, a greater number of people will become infected later in life (ages 10-30 years) with symptomatic IM when compared to other populations.2
The highest incidence of symptomatic IM occurs between the ages of 15 and 24 years. Overall, the incidence in the United States is roughly 500 cases per 100,000 persons per year.1 The incidence of symptomatic infection is approximately 30 times higher in whites than in blacks in the U.S.4 This difference may reflect earlier exposures to EBV along with asymptomatic infection acquired at younger ages among blacks. It does not appear that the incidence of IM is seasonal in nature or that one gender tends to be more predisposed than the other.2
IM is most common in populations with many young adults in close proximity, such as college students. Approximately 30% to 75% of college freshmen are seropositive for EBV. Each additional year, another 10% to 20% of students will become infected. Only 30% to 50% of these infected individuals will develop symptomatic IM, with 1% to 3% of all college students becoming infected each year.2,5
Pathogenesis
EBV replicates within oropharyngeal epithelial cells. The virus is then released into oropharyngeal secretions and infects B cells in the oropharyngeal lymphoid tissues. Once B cells are infected, dissemination of infection throughout the lymph system occurs, and EBV may incubate there for 30 to 50 days before clinical symptoms appear.1 Cytotoxic T lymphocytes become activated to control both acute and reactivated disease. EBV may be shed continually from the oropharynx for up to a year following primary infection. EBV is typically a lifelong infection that latently persists in memory B cells where it may be reactivated and shed into oropharyngeal secretions.2
Transmission
Transmission occurs less than 10% of the time when a susceptible individual comes into close contact with an EBV shedder; therefore, it is not a particularly contagious disease. Virus can be shed in salivary secretions from patients with IM for many weeks. The median duration of virus shedding is 32 weeks after diagnosis, although some studies show that the EBV virus could be shed for decades.6,7
Precautions are not needed to prevent transmission because of the high percentage of people who are already seropositive for EBV. Transmission primarily occurs through contact with infected saliva, whether via kissing (the “kissing disease”) or less commonly through sexual contact, blood transfusions, or by sharing utensils.4
Signs and Symptoms
Young children infected with EBV generally are asymptomatic or have such mild disease that symptoms are not recognized. Older children, teens, and young adults are more likely to develop the symptomatic clinical signs of IM. Once infected, some patients may experience a prodromal period of symptoms where they may experience headache, anorexia, and fatigue for 1 to 2 weeks before more classical symptoms become apparent.4
Classical symptoms of IM include pharyngitis, moderate-to-high fever, and generalized lymphadenopathy in the inguinal, axillary, posterior auricular, and cervical nodes.1 Additionally, patients commonly experience fatigue that may be severe. Various other symptoms are categorized in TABLE 1.1,2,4
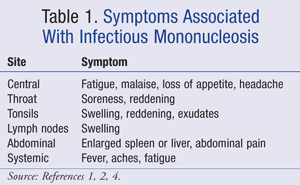
Pharyngitis may produce pharyngeal inflammation and exudates. Pharyngitis can be caused by various bacteria (e.g., group A streptococcus) and viruses (EBV, adenovirus, influenza, herpes simplex), so excluding these causes for symptoms may be pertinent. Throat culture testing for group A streptococcus is likely the most common test to exclude before diagnosis of IM.
Diagnosis
Patients with IM commonly complain of sore throat and fatigue. These symptoms, along with fever and lymphadenopathy, often are the basis for diagnosis alone; however, Hoagland’s criteria may be commonly used to help diagnose IM.2,8,9 These criteria cite a 50% lymphocyte count with at least 10% atypical lymphocytes in the presence of fever, pharyngitis, and adenopathy with a positive serologic test as being diagnostic for IM.9
Around 50% of patients also develop splenomegaly by the second week. Splenic rupture is a rare but potentially life-threatening complication. It is more common in males, possibly due to the higher rates of participation in contact sports, although splenic rupture is spontaneous in roughly half of the cases.10 Splenic rupture typically occurs in the first 3 weeks of symptomatic disease and may be detected by a decreasing hemoglobin level or the presence of abdominal pain.
Pharyngitis, however, is a common complaint of various infections so other viral or bacterial causes need to be ruled out, such as the common cold or influenza.2 Differentiation is important because an infection with group A streptococcus, the most common bacterial cause of pharyngitis, may require antibiotic treatment.2 IM does not require antibiotic treatment since it is caused by a viral infection. If a patient with IM is treated with amoxicillin or ampicillin, a morbilliform rash will likely develop. A patient presenting with a morbilliform rash after taking a penicillin antibiotic for pharyngitis should be assessed for IM.1 Splenomegaly and fatigue are the two chief symptoms that distinguish IM from bacterial pharyngitis.
Diagnostic laboratory tests include a positive heterophile antibody test (Monospot) and the presence of atypical lymphocytes on a peripheral-blood smear. Antibodies may remain positive for 9 months after the onset of IM, so a positive Monospot test may not indicate an acute infection of IM.11 In most adolescents, a diagnosis of IM can be confirmed on the basis of the clinical presentation along with a positive Monospot.2,8
Complications
For the majority of patients, IM runs a self-limiting course and recovery occurs without sequelae, although complications can occur.8 Hematologic complications are the most common, occurring in 25% to 50% of all cases of IM. These complications may include hemolytic anemia, thrombocytopenia, thrombotic thrombocytopenic purpura, and disseminated intravascular coagulation.2 These complications are generally characterized as mild due to the slight dip in laboratory values, which typically return to normal within a month, whereas severe complications, such as aplastic anemia requiring bone marrow transplantation, are rare.2 Neurologic complications occur in 1% to 5% of cases and can include Guillain-Barré syndrome, facial-nerve palsy, meningoencephalitis, aseptic meningitis, transverse myelitis, peripheral neuritis, cerebellitis, and optic neuritis.2 Other rare, but potentially life-threatening complications include upper airway obstruction and X-linked lymphoproliferative disease (XLP). Males with XLP syndrome have an inability to mount an immune response to EBV because of an X-chromosome mutation, which results in very severe or fatal IM.2,8 This disorder can be diagnosed prenatally, and early bone marrow transplantation is an option to correct the disease.2
Persistent fatigue lasting for 6 months or longer with functional impairment can also occur.2 Female gender and greater fatigue severity at symptom onset demonstrate a higher risk of developing chronic fatigue syndrome (CFS).
Pharmacologic Treatment
Recommended therapy for management of IM has not changed much over the years. Pharmacologic options remain limited and mostly include symptomatic treatment or supportive care. Fever, myalgias, and throat discomfort can be treated with nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen.2,8 Throat lozenges and sprays containing benzocaine or phenol along with prescription lidocaine 2% can be used for sore throat.1,4
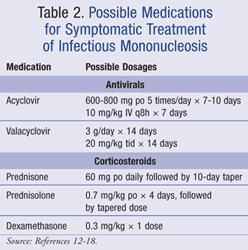
Acyclovir is an antiviral medication that inhibits DNA polymerase of EBV. Acyclovir’s effects on IM have been studied in double-blind, placebo-controlled trials and have demonstrated a suppression in the shedding of EBV in the saliva of infected patients, but EBV replication resumed after treatment was discontinued.12,13 The use of acyclovir, however, provided no significant reductions on individual clinical symptoms of duration of fever, lymphadenopathy, hepatomegaly, splenomegaly, weight loss, or tonsillar swelling. A meta-analysis of controlled trials assessing acyclovir’s role in the treatment of IM failed to show a clinical benefit when compared to placebo and stated that clinical data do not support the use of acyclovir for IM.14
Valacyclovir has also been studied in patients with IM. A total of 20 college students were treated with valacyclovir or placebo for 14 days. Results showed small decreases in severity of illness scores at day 15 and a reduction of EBV excretion in saliva; however, this was a small trial with questionably clinically significant results.15
Reports have demonstrated that antivirals may decrease EBV shedding in saliva of treated patients, but since most people are EBV-seropositive from previous exposure, special precautions against transmission from symptomatic patients are not necessary.1,2 Since data supporting clinical benefit are lacking, antiviral use is not routinely recommended.
Corticosteroids have also traditionally been used for IM to prevent airway obstruction and lessen pharyngeal inflammation. The role of corticosteroids in the symptomatic treatment of IM was assessed by treating with prednisone 60 mg daily tapered over 10 days. Results showed that fever and lymphadenopathy resolved slightly faster with corticosteroids compared to placebo, but clinical significance was questioned.16
The combination of acyclovir 800 mg orally 5 times per day and prednisolone 0.7 mg/kg for 4 days with subsequent dose tapering of 0.1 mg/kg/day over 6 days has also been studied. The effectiveness of combination therapy was compared to acyclovir monotherapy in a double-blind, placebo-controlled trial of 94 patients.17 This trial showed that the addition of prednisolone had no significant effect on duration of general illness, sore throat, weight loss, or absence from school or work.
A meta-analysis was conducted to assess the efficacy of corticosteroids in IM and included four trials utilizing only corticosteroids and three trials using corticosteroids plus acyclovir.18 This meta-analysis concluded that no benefit was seen in 8 of 10 health assessment measures. Two of the seven trials found a reduction in sore throat pain within 12 hours with corticosteroids, but this benefit was not maintained. The overall conclusion was that there was insufficient evidence to recommend corticosteroids for symptom control in IM.18
Furthermore, corticosteroids have not been proven to reduce disease complications, rates of hospital admission, or length of hospital stay.1,8 Currently, corticosteroids are only recommended for life-threatening complications of IM such as acute upper airway obstruction, severe thrombocytopenia, and severe hemolytic anemia.1,2 Unfortunately, data on the dosing and duration of corticosteroids for these indications are not available. Possible treatment options for IM are listed in TABLE 2.12-18
Nonpharmacologic Treatment
Nonpharmacologic treatment is an essential part of managing IM. The mainstay of therapy includes restriction of activity. Adequate rest is important, but bed rest is not required.2 It is also important to stay hydrated and maintain adequate nutrition because IM may lead to a decrease in appetite. Maintaining adequate hydration is even more important in individuals taking NSAIDs for symptomatic relief to avoid renal insufficiency.
Common Concerns
When to Return to School/Work: Patients with IM may return to school or work as soon as they are feeling better. IM is not a highly contagious disease, and most people have already been infected with EBV. In addition, patients with IM are typically infected and contagious for 4 to 8 weeks before symptoms appear. It is important to note that patients may experience fatigue and may have to ease back into their normal schedules.
Avoiding Splenic Rupture: As previously mentioned, splenomegaly is a common symptom encountered, rarely leading to splenic rupture. Abdominal pain and decreasing hemoglobin are hallmark signs of splenic rupture. The exact cause of splenic rupture is unknown but might be secondary to lymphocytic infiltration of the spleen that disrupts the normal tissue anatomy and support structures, leaving the spleen fragile.19 If splenic rupture does occur, a splenectomy may be required. Roughly half of all cases of splenic rupture occur spontaneously, but concern for traumatic damage remains. Since IM largely affects teenagers and young adults, many of whom participate in sports and other physical activities, participation in these activities should likely be avoided.
The question may arise as to when a patient with IM may return to sports or other physical activities. A case review of splenic rupture in athletes with IM showed that almost all patients had splenomegaly presenting between days 4 and 21 of illness onset and splenic rupture occurring between 4 and 7 weeks after illness onset.3,20 The presence of splenomegaly on physical examination may help reinforce the recommendation of activity restriction, but this condition is not always identified on physical examination, leading to the possible need for radiographic evaluation with ultrasound scans.
There are no specific guidelines that state when a patient with IM may return to athletic participation, but the general recommendation is roughly 3 weeks after initial symptom onset for athletes to resume activity in noncontact sports and at least 4 weeks after symptom onset for strenuous contact sports or activities that can lead to increased abdominal pressure (e.g., football, hockey, gymnastics, basketball, weight lifting), provided that splenomegaly is not identified on physical examination. In the case of high contact or collision sports, a radiologic evaluation of the spleen might be warranted before return to activities.20-23 It must be pointed out that athletes will likely have to initiate training at reduced levels and gradually increase activities as tolerated secondary to the fatigue they will likely be experiencing. It may take several months for athletes to regain a pre-illness level of fitness.24
Chronic Fatigue: Whereas resolution of acute symptoms of IM is typical in 1 to 2 weeks, fatigue may linger for months.25 CFS is clinically characterized by severe disabling fatigue and a combination of symptoms that features self-reported impairments in concentration and short-term memory, sleep disturbances, and musculoskeletal pain.26 CFS is a diagnosis only after other causes have been excluded.
A study by Katz et al examined the course and outcome of CFS in 301 adolescents aged 12 to 18 years over a 2-year period following IM.27 Results indicated that at 6 months post IM, approximately 13% of adolescents had CFS, and at 12 and 24 months post IM, 7% and 4% of patients still had CFS, respectively. This study showed that female gender was associated with a greater incidence of CFS.
Role of the Pharmacist
Pharmacists can play a key role in the management and education of IM. The appearance of a morbilliform rash after penicillin administration for pharyngitis may signal that IM is the cause of the pharyngitis. Although prescription agents are not usually necessary for IM, counseling on selection of NSAIDs or acetaminophen for supportive care may be needed. Providing answers to commonly asked questions about avoidance of sports and fatigue may also be needed.
REFERENCES
1. Infectious mononucleosis. EBSCO DynaMed [online database]. www.ebscohost.com/dynamed. Accessed February 1, 2013.]
2. Luzuriaga K, Sullivan J. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000.
3. Maki DG, Reich RM. Infectious mononucleosis in the athlete. Am J Sports Med. 1982;10:162-173.
4. Aronson MD, Auwaerter PG. Infectious mononucleosis in adults and adolescents. In: Hirsch MS, Kaplan SL, eds. UpToDate. Waltham, MA: UpToDate; 2013. www.uptodate.com. Accessed February 1, 2013.
5. Haines JD. When to resume sports after infectious mononucleosis: how soon is safe? Postgrad Med. 1987;81:331-333.
6. Balfour HH Jr, Holman CJ, Okanson KM, et al. A
prospective clinical study of Epstein-Barr virus and host interactions
during acute infectious mononucleosis. J Infect Dis. 2005;192:1505-1512.
7. Vetsika EK, Callan M. Infectious mononucleosis and Epstein-Barr virus. Expert Rev Mol Med. 2004;6:1-16.
8. Vouloumanou EK, Rafailidis PI, Falagas ME. Current diagnosis and management of infectious mononucleosis. Curr Opin Hematol. 2012;19:14-20.
9. Hoagland RJ. Infectious mononucleosis. Prim Care. 1975;2:295-307.
10. Gayer G, Zandman-Goddard G, Kosych E, Apter S.
Spontaneous rupture of the spleen detected on CT as the initial
manifestation of infectious mononucleosis. Emerg Radiol. 2003;10:51-52.
11. Peter J, Ray CG. Infectious mononucleosis. Pediatr Rev. 1998;19:276-279.
12. Andersson J, Britton S, Emberg I, et al. Effect of
acyclovir on infectious mononucleosis: a double-blind,
placebo-controlled study. J Infect Dis. 1986;153:283-290.
13. van der Horst C, Joncas J, Ahronheim G, et al. Lack of
effect of peroral acyclovir for the treatment of infectious
mononucleosis. J Infect Dis. 1991;164:788-792.
14. Torre D, Tambini R. Acyclovir for treatment of infectious mononucleosis: a meta-analysis. Scand J Infect Dis. 1999;31:543-547.
15. Balfour HH, Hokanson KM, Schacherer RM, et al. A virologic pilot study of valacyclovir in infectious mononucleosis. J Clin Virol. 2007;39:16-21.
16. Brandfonbrener A, Epstein A, Wu S, Phair J.
Corticosteroid therapy in Epstein-Barr virus infection effect on
lymphocyte class, subset, and response to early antigen. Arch Intern Med. 1986;146:337-339.
17. Tynell E, Aurelius E, Brandell A, et al. Acyclovir and
prednisolone treatment of acute infectious mononucleosis: a
multicenter, double-blind, placebo-controlled study. J Infect Dis. 1996;174:324-331.
18. Candy B, Hotopf M. Steroids for symptom control in infectious mononucleosis. Cochrane Database Syst Rev. 2006;(3):CD004402.
19. Burroughs KE. Athletes resuming activity after infectious mononucleosis. Arch Fam Med. 2000;9:1122-1123.
20. Johnson MA, Cooperberg PL, Boisvert J, et al. Spontaneous splenic rupture in infectious mononucleosis. Am J Roentgenol. 1981;136:111-114.
21. Eichner ER. Infectious mononucleosis. Phys Sportsmed. 1996;24:49-54.
22. Oski FA. Management of a football player with mononucleosis. Pediatr Infect Dis J. 1994;13:938-939.
23. Waninger KN, Harcke HT. Determination of safe return
to play for athletes recovering from infectious mononucleosis: a review
of the literature. Clin J Sport Med. 2005;15:410-416.
24. Noffsinger J. Physical activity considerations in children and adolescents with viral infections. Pediatr Ann. 1996;25:585-589.
25. Hickie I, Davenport T, Wakefield D, et al.
Post-infective and chronic fatigue syndromes precipitated by viral and
non-viral pathogens: prospective cohort study. BMJ. 2006;333:575.
26. Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953-959.
27. Katz BZ, Shiraishi Y, Mears CJ, et al. Chronic fatigue syndrome after infectious mononucleosis in adolescents. Pediatrics. 2009;124:189-193.
To comment on this article, contact rdavidson@uspharmacist.com.





