US Pharm. 2021;46(8):28-33.
ABSTRACT: Atopic dermatitis, or eczema, is a chronic inflammatory skin disease that is common in the pediatric population, with 90% of patients experiencing an eruption by age 5 years. Atopic dermatitis is identified by its hallmark pruritic rash and involves a cycle of flare-up and remission. Management with nonpharmacologic and pharmacologic options can lessen the symptoms of atopic dermatitis and improve the patient’s quality of life. The mainstay of treatment is topical agents, but more severe cases may require additional therapies. Pharmacists are highly accessible and therefore are positioned to assist parents and caregivers in learning about treatment options for pediatric atopic dermatitis.
Atopic dermatitis (AD), or eczema, is a chronic inflammatory skin disease that typically presents in childhood, although it sometimes develops in adulthood.1 Approximately 31.6 million (10%) Americans—9.6 million of them younger than age 18 years—have AD. Ninety percent of patients experience an AD eruption by age 5 years, with about 60% of these cases occurring during the child’s first year of life. An estimated one-third of children have moderate-to-severe disease. AD occurs equally in all skin types and ethnicities, and prevalence is similar in girls and boys. The disease resolves by adulthood in the majority (70%) of patients.1
AD, which is identified by its hallmark pruritic rash, involves a cycle of flare-up and remission.1 Pruritus is the primary indicator of disease burden, but patients may also present with erythema, edema, excoriation, or lichenification. Serum immunoglobulin E levels may be elevated in AD. There is also a link between AD and the presence or family history of asthma and/or allergic rhinitis.1
The mainstay of AD treatment is topical agents, and more severe cases may also require phototherapy or systemic agents.2 Several topical agents from different pharmacologic classes may be used in combination to address different aspects of AD pathogenesis.2 Active exacerbation of AD, known as a flare, requires intensive treatment; when the flare is in remission, preventive treatment is used.
FLARE TREATMENT
Primary treatment of AD flares aims to reduce inflammation and blunt the immune response in order to prevent further inflammatory mediation. Some treatment options, such as moisturizers, work by strengthening the skin barrier. Although on their own those therapies are rarely sufficient to resolve a disease flare, they are an important part of flare management.3
First-Line Therapy
First-line therapy for AD includes moisturizers, topical corticosteroids (TCS), and topical calcineurin inhibitors (TCIs).4
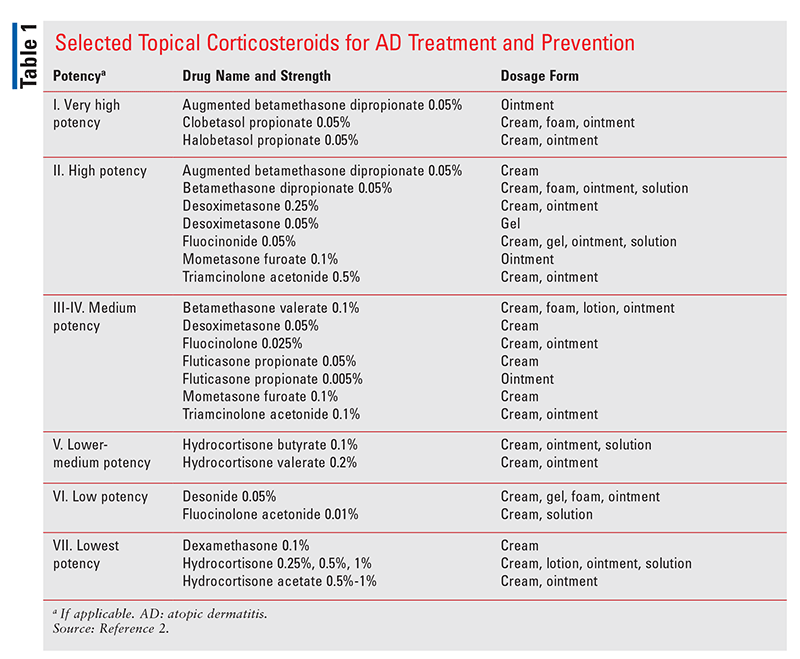
Topical Inflammatory Agents: TCS are recommended for patients who do not respond to proper skin care and regular use of moisturizers.2 As comparison data are lacking on the many available TCS, any agent in the class may be used. Selection may be based on availability or potency (TABLE 1). TCS may be classified by potency, with class I comprising the highest-potency drugs and class VII comprising those with lowest potency. The frequency and severity of adverse effects (AEs) are directly correlated to drug potency; therefore, in choosing TCS, a balance is needed between drug potency and symptom control, particularly in pediatric patients. During a flare, a medium- or high-potency TCS is typically initiated. The selected agent is applied over the lesion or lesions until resolution, after which the potency is reduced or the TCS is discontinued.2 In general, low-potency agents are preferred for the face, neck, and skin folds, whereas moderate-potency agents may be appropriate for the trunk and extremities.2
In patients with significant flares (i.e., severe AD), wet-wrap therapy may be used to quickly reduce AD severity.5 This method involves the use of a topical agent covered by a wetted layer of bandages and followed by a dry outer layer of bandages. The proposed mechanism is that the wet layer increases the occlusion of the topical agent, increasing penetration into the skin and reducing water loss. Clinical trials have shown that TCS wet wraps were more efficacious than moisturizer-only wet wraps. Because of the increased risk of hypothalamic-pituitary-adrenal axis suppression, low- and medium-potency corticosteroids are preferable with this method.5
Systemic corticosteroids are not routinely recommended in AD based on the increased risk of AEs, including rebound flare and increased disease severity, and a lack of studies showing benefits.4
TCIs: TCIs, a second class of anti-inflammatory therapy, are immunosuppressants that blunt the propagation of inflammation by inhibiting T-cell function.1,3 These agents are indicated for use in patients with AD who are not immunocompromised and have failed to respond to other topical treatments or in whom other treatments are not appropriate.1 TCIs have a milder AE profile than corticosteroids, with little potential for systemic absorption and immunosuppression.3 Skin atrophy is uncommon with TCI therapy, and common AEs associated with TCIs are burning and itching of the skin.1 TCIs carry a black box warning for cancer risk; however, this relationship is poorly documented in studies.6
Two TCI agents, tacrolimus and pimecrolimus, are currently available. Tacrolimus, an ointment, is available in two strengths: 0.03% (approved for patients aged 2 years and older) and 0.1% (for those aged 16 years and older).7 In one meta-analysis, tacrolimus had efficacy similar to that of medium-potency TCS, and it improved symptoms such as erythema, pruritus, and sleep loss.8 Tacrolimus is more potent than pimecrolimus, making it a better choice during AD flares.2 Pimecrolimus, which is available as a 1% cream, is approved for patients aged 2 and older.9
Second-Line Therapy
Phototherapy: Phototherapy with ultraviolet (UV) radiation is a second-line treatment option for patients with AD who have failed first-line therapy; it may also be used as maintenance therapy.4 Multiple types of UV therapy are available, including natural sunlight, narrowband, broadband, narrowband-broadband combination, and topical and systemic psoralen combined with these modalities. Based on the lack of head-to-head data, no single phototherapy modality is preferred over others. Topical immunosuppressants such as TCIs should be avoided in UV therapy; however, moisturizer and TCS use may be continued. UV therapy carries a minimal risk of skin cancer, but more commonly reported AEs are nausea, fatigue, and dermatologic reactions.4
Antimicrobials and Antiseptics: Skin infections, both bacterial and viral, can play a role in AD flares. Children with poorly controlled AD are at higher risk for infection owing to a compromised skin barrier and dysregulated immune response.2 Staphylococcus aureus and herpes simplex virus (HSV) are the most commonly encountered pathogens.2,4 Treatment of underlying infections can improve skin conditions and symptoms of AD flares. Empiric antimicrobial therapy should cover S aureus and Streptococcus based on the prevalence of these organisms; however, cultures may be obtained in severe cases.3 Systemic antibiotics are reserved for patients with clear clinical evidence of infection.4
Bleach Baths: This therapy may be recommended for children with moderate-to-severe AD flares and clinical signs of bacterial infection.2 Dilute bleach baths at a strength of 0.0005% bleach have shown to be effective in AD patients without contributing to antimicrobial resistance.3,10 This concentration is produced by adding 120 mL of 6% household bleach to a full bathtub (40 gallons) of water. Bleach baths may be used for 5 to 10 minutes twice weekly.3 In a meta-analysis, commonly reported AEs of bleach baths and cleansers were stinging/burning, erythema, urticaria, and oozing; importantly, there was no significant difference in AE frequency for bleach baths versus water baths.10 Although bleach baths are effective in reducing AD severity, clinical support is lacking, and they have not been found to be more effective than water baths alone.10
Topical Phosphodiesterase (PDE) Inhibitors: The agent crisaborole is a PDE4 inhibitor that prevents the release of inflammatory cytokines by increasing intracellular cyclic adenosine monophosphate.11,12 This topical formulation (ointment) avoids the gastrointestinal AEs associated with the systemic PDE4 inhibitors used to treat psoriasis.11 Crisaborole is approved for use in mild-to-moderate AD in children aged 3 months and older.12 Guidance on its place in therapy has not been recommended, but two clinical trials excluded patients who had used TCS or TCI within 14 days prior to therapy initiation.11
Systemic Immunosuppressants and Biologics: Immunosuppressants and biological agents are of particular interest in novel drug development. Systemic immunosuppressants target several components of the immune system in order to prevent activation and modulation of inflammatory signaling pathways. They do not restore skin-barrier function, but they limit further immune responses that cause inflammation and itching.7 Because of their cost and safety profile, immunosuppressant agents are reserved for AD patients who remain symptomatic despite optimized topical regimens or phototherapy and patients who are unresponsive to other therapies.4 Immunosuppressants are administered in combination with hydration and emollient therapy, and cyclosporine, azathioprine, methotrexate, and mycophenolate mofetil are commonly used.4,7
In 2020, the biological agent dupilumab was approved for treatment of moderate-to-severe and poorly controlled AD in pediatric patients aged 6 years and older.13 Administered as a biweekly injection, this drug inhibits interleukin (IL)-4 and IL-13, and it is often used in combination with TCS. Studies have shown improvements in resolution of symptoms such as itching, sleep disturbances, and anxiety.13-15 Common AEs include conjunctivitis, keratitis, injection-site reaction, and HSV infection.13
Antihistamines: The use of systemic antihistamines to treat AD is not widely supported by current data and guidelines.4 Although antihistamines do not have a role in resolving AD, they may be useful in managing pruritus and preventing skin damage caused by scratching.3,4 Antihistamines with sedating effects, such as diphenhydramine and hydroxyzine, are more effective in resolving sleep disturbances related to pruritus, but they should be used with caution in infants and young children owing to the risk of serious AEs and paradoxical reaction.3 The additives in topical antihistamines can potentially cause further skin irritation and are not effective in controlling AD-related pruritus; therefore, these formulations should be avoided.3
FLARE PREVENTION
During nonactive AD, the goal is to prevent future flares, or relapses.5 The method used to prevent recurrence differs depending on the individual patient, along with the frequency, severity, and site of the patient’s AD. Flare prevention focuses primarily on protecting the skin barrier and limiting exposure to triggers. Some patients respond well to the use of moisturizers only, whereas others benefit from regular use of TCS or TCIs.5
Nonpharmacologic Interventions
Moisturizers: Moisturizers are considered the cornerstone of AD management and should be included in all treatment plans.2,5 Transepidermal water loss and xerosis (excessive dryness) resulting from skin-barrier disruption are common in AD.2,7 The use of moisturizers has been shown to lengthen the time to first flare.5 Moisturizers should be applied to the entire body at least once daily, preferably soon after bathing, regardless of whether dermatitis is present.2
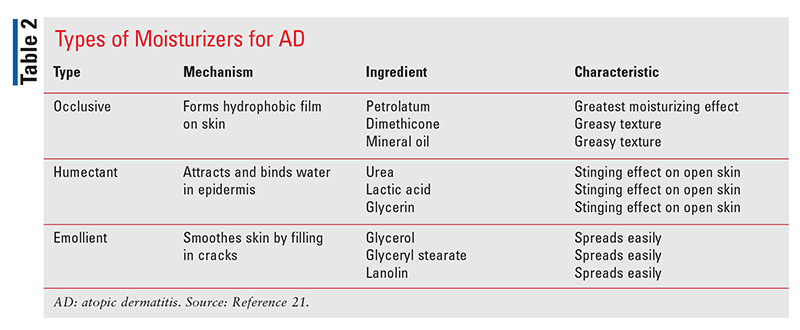
Moisturizers come in several different formulations: occlusive, humectant, and emollient (TABLE 2). Occlusive ointments have the greatest moisturizing effect but are sometimes avoided because of their greasy feel.2 Creams can be less greasy than ointments, but most contain preservatives that may cause a burning or stinging sensation on broken skin. Lotions have the highest proportion of water, but because of evaporation they have the least moisturizing effect.2 Pure oil products, such as coconut oil, are not recommended based on their potential to dry out the skin and increase transepidermal water loss.16 Moisturizers used for AD should be fragrance free and contain the lowest amount of preservatives, which have irritative properties.7 The daily application of moisturizers creates a protective barrier on the skin and can help reduce the need for prescription anti-inflammatory treatments.2,5
Bathing: Bathing is an important part of maintenance skin care for patients with AD, but it must be done properly.2 Water serves to hydrate the skin; however, if left to evaporate, it can increase the occurrence of transepithelial water loss, which underscores the importance of applying moisturizers immediately after bathing. No data exist on showering versus soaking, so patient preference for bathing type may be followed, but the water should be warm. The recommended frequency is daily for about 5 to 10 minutes. If significant inflammation is present, soaking in plain water for 20 minutes is advised, followed by application of pharmacologic anti-inflammatories immediately afterward, without toweling dry. Soap can damage and dry the skin, but non–soap-based cleansers may be used if they are low in pH, hypoallergenic, and fragrance free.2 The clinical benefit of adding emollients, oils, or other additives to the bath water remains unevaluated.2
Trigger Avoidance: Triggers are not always avoidable; however, it is important to limit patient exposure whenever possible.5 Known mechanical, chemical, and other irritants (e.g., wool, acids, excessive heat) should be avoided. Patients with dust-mite allergy may benefit from using pillow and mattress covers.5
Educational Interventions
Owing to the need for multiple treatments and the complex pathogenesis, patients or caregivers should be educated about various aspects of AD.5 Education should include disease mechanisms, pertinent therapies, and management goals and be geared to improving treatment adherence. Evidence-based options include multidisciplinary educational programs, workshops, nurse-led educational sessions, parental video instruction, written action plans, and psychological interventions. Video instruction was found to be less time-consuming and more effective than written pamphlets in improving AD symptoms and severity. Psychological interventions include autogenic training, biofeedback, brief dynamic psychotherapy, cognitive-behavioral therapy, habit-reversal behavioral therapy, and stress-management programs. All of these interventions may provide patients or caregivers with coping mechanisms to help manage AD.5
Food-Allergy Testing
Many AD patients, especially those with severe disease, present with food allergies.5 The influence of food allergies on AD is not entirely understood, as these allergies more frequently induce an immediate type I immune response and may or may not exacerbate AD. An expert panel sponsored by the National Institute of Allergy and Infectious Diseases suggested that food-allergy testing be considered in patients younger than age 5 years with moderate-to-severe AD and either persistent disease despite optimal therapy or reliable history of immediate allergic reaction after ingesting a specific food.17 If tested and confirmed, the food (or foods) potentiating AD should be avoided. Avoidance diets should not be initiated in AD patients based solely on suspicion of a food allergy, given the possibility of weight loss, poor growth, and vitamin deficiencies.17 A diagnostic elimination diet may be performed if AD symptoms consistently worsen upon ingestion of a specific food. If symptoms subside after the food is removed from the diet, an oral food challenge may be performed under the supervision of an allergist.5
Dietary Interventions
Probiotics: The intestinal microbiota differs in persons with and without AD.5 Probiotics modify the microbiota and could change the host immune response.6 A Cochrane review of 12 trials found no significant improvements in AD symptoms or disease severity in patients given probiotics versus placebo.18 The use of probiotics such as Lactobacillus for flare treatment or prevention is not currently recommended.5
Other Supplements: Although fatty-acid deficiencies have been proposed to play a role in AD, fish oil has not been shown beneficial for AD management.5 Evening primrose oil and borage oil have been suggested for AD management because of possible anti-inflammatory properties based on their gamma-linolenic acid content; however, studies have not shown a proven benefit.5 Results of a Cochrane review and a systematic review confirm the lack of evidence for the use of vitamins and minerals in AD.19,20
Pharmacologic Interventions
When monotherapy moisturizer therapy is not effective for preventing a patient’s flares, the use of TCS one to two times per week or TCIs two to three times per week is recommended in addition to daily moisturizers.5 Currently, no single drug class is preferred over the others, as no head-to-head comparisons are available. Long-term use of these agents presents some safety concerns. Long-term TCS use has led to higher rates of viral infections; upper respiratory infections; and ear, nose, and throat symptoms, and use beyond 44 weeks has not been evaluated. Long-term TCI use has been associated with increases in skin infections, application-site reactions, and nasopharyngitis.5
THE PHARMACIST’S ROLE
Pharmacists can play a significant role in the selection of nonpharmacologic therapy and in making recommendations on pharmacologic options for both prevention and management of AD flares in pediatric patients. Counseling and education are important aspects of AD management that outpatient and community pharmacists can provide given their high accessibility. AD is a chronic inflammatory disorder, but pharmacists can help reduce the disease burden and improve quality of life for their pediatric patients.
REFERENCES
1. Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338-351.
2. Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116-132.
3. Tollefson MM, Bruckner AI, Section on Dermatology. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134(6):e1735-1744.
4. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327-349.
5. Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71(6):1218-1233.
6. Ohtsuki M, Morimoto H, Nakagawa H. Tacrolimus ointment for the treatment of adult and pediatric atopic dermatitis: review on safety and benefits. J Dermatol. 2018;45(8):936-942.
7. Lyons JJ, Milner JD, Stone KD. Atopic dermatitis in children: clinical features, pathophysiology, and treatment. Immunol Allergy Clin North Am. 2015;35(1):161-183.
8. El-Batawy MM, Bosseila MA, Mashaly HM, Hafez VS. Topical calcineurin inhibitors in atopic dermatitis: a systematic review and meta-analysis. J Dermatol Sci. 2009;54(2):76-87.
9. Elidel (pimecrolimus) package insert. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; March 2014.10. Chopra R, Vakharia PP, Sacotte R, Silverberg JI. Efficacy of bleach baths in reducing severity of atopic dermatitis: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2017;119(5):435-440.
11. Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75(3):494-503.
12. Eucrisa (crisaborole) package insert. New York, NY: Pfizer Inc; April 2020.
13. Dupixent (dupilumab) package insert. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; June 2021.
14. Cline A, Berg A, Bartos GJ, et al. Biologic treatment options for pediatric psoriasis and atopic dermatitis—a review. J Clin Aesthet Dermatol. 2020;13(6 suppl):33-38.
15. Paller AS, Siegfried EC, Thaci D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;88(5):1282-1293.
16. Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657-682.
17. Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
18. Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for the treatment of eczema: a systematic review. Clin Exp Allergy. 2009;39(8):1117-1127.
19. Vaughn AR, Foolad N, Maarouf M, et al. Micronutrients in atopic dermatitis: a systematic review. J Altern Complement Med. 2019;25(6):567-577.
20. Bath-Hextall FJ, Jenkinson C, Humphreys R, Williams HC. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012;(2):CD005205.
21. Rawlings AV, Canestrari DA, Dobkowski B. Moisturizer technology versus clinical performance. Dermatol Ther. 2004;17(suppl 1):49-56.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





