US Pharm. 2023;48(1):30-36.
Affecting more than 3 million Americans, glaucoma is a group of optic degenerative neuropathies that can lead to irreversible vision impairment and blindness. It is associated with degradation of ganglion cells and retinal nerve fibers in the eye, resulting in intraocular pressure (IOP)–related damages to the optic nerve, which is located behind the eyes. Glaucoma is categorized into primary and secondary glaucoma, both of which have two primary subtypes: primary open-angle glaucoma (POAG) and primary angle-closure glaucoma (PACG).
In POAG, the angle of the eye—between the iris and cornea—remains open; however, aqueous flow through the trabecular meshwork and uveoscleral routes is diminished, which can ultimately result in an increase in IOP. This increase in pressure can potentially cause mechanical stress and strain on the posterior structures of the eye and can also lead to changes in blood flow to the optic nerve, decreasing oxygen and nutrient supply. All of this can lead to retinal ganglion cell death. However, almost one-half of patients with POAG have an IOP that is considered within normal range (12 mmHg-22 mmHg) at diagnosis, emphasizing the importance of not just relying on IOP measurements as the sole diagnostic tool. In the presence of normal IOP, this condition has been referred to as normal-pressure glaucoma. POAG occurs when there is partial blockage or complete closure of the drainage structure of the eye called the trabecular meshwork by the peripheral iris, leading to increased IOP and optic nerve damage.1-3
POAG is the predominant subtype, affecting almost 2.7 million Americans aged 40 years and older. Patients diagnosed with POAG report experiencing a decreased quality of life, with difficulty performing daily functions, such as driving, and have higher incidences of reported falls and motor vehicle accidents. In 2015, the economic burden of glaucoma alone on the American economy was estimated to be $2.9 billion, and the cost of treating and preventing glaucoma is about $5.8 billion per year in the United States.4,5
Glaucoma is a major health concern; it is the second leading cause of blindness, after cataracts. There is currently no cure for glaucoma to undo vision damage, and lifelong treatment is usually required in the management of this condition. There are different classes of medications utilized to help decrease IOP, but adherence to such therapies is often lacking, with a range anywhere from 5% to 80% across studies. Pharmacists are essential healthcare workers who provide patients and caregivers with educational resources for their medications to prevent any potential medication-related issues on administration, adherence, and therapy, ensuring all patients are receiving the most beneficial outcomes.6
Risk Factors
The direct cause of glaucoma is unknown, but a mixture of environmental, hereditary, and vascular factors was shown to have a significant influence on the risks of developing glaucoma. TABLE 1 lists some of the common risk factors that make certain people more prone to developing the disease. The main risk factor for glaucoma is older age. Prevalence was found to be less than 1% for POAG in patients younger than age 55 years, close to 2% in patients aged 56 to 65 years, and in patients closer to 80 years of age, the risk of developing open-angle glaucoma was 4%. Older age was also shown to have a link with an increased risk of blindness in patients with POAG. Alongside age, race was shown to be an important risk factor for glaucoma: patients of African American, Asian, as well as Native American descent are more likely to develop the disease compared with Caucasians.3,7-10
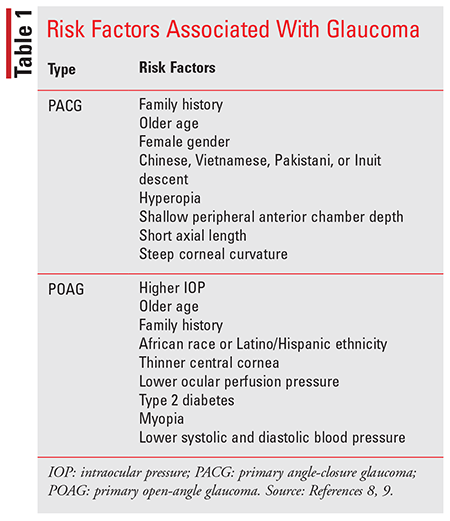
Patients who have a family history of glaucoma are more likely to develop it later in life. Studies have shown first-degree relatives have an approximate ninefold increase in developing POAG, with this risk increasing with the number of relatives diagnosed. Other comorbid conditions such as diabetes, heart disease, and hypertension can cause glaucoma, especially if the conditions are poorly managed. Diabetic patients’ risk for glaucoma development is increased by 5% each year after their diagnosis as compared with patients without diabetes. Hypertension is noted to be associated with an increase in IOP, which raises the risk of developing glaucoma. Physical injuries to the eye can cause trauma and cause damage to the optic nerve, leading to glaucoma. Other eye conditions can make glaucoma more likely to occur. Eye inflammation, retinal detachment, and eye tumors can potentially be other risk factors. Most of the signs and symptoms associated with glaucoma are difficult to notice, so it is important that all patients who are at risk for glaucoma have their eyes routinely checked.3,7-9,11
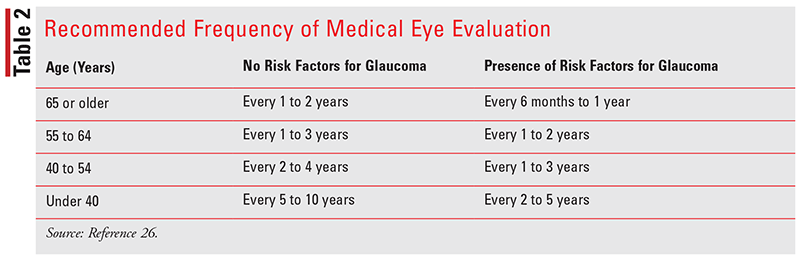
Screening of the general population for glaucoma is not recommended; however, the American Academy of Ophthalmology suggests scheduling an eye exam every 1 to 3 years after age 40 years; 1 to 2 years after age 55 years; and every 6 months to 1 year at age 65 years and older if at risk for glaucoma (see TABLE 2).
Clinical Presentation
Most forms of glaucoma present with no warning signs, making it impossible to notice a change without going to an ophthalmologist for an eye examination. Patients do not usually suffer visual field defects until approximately 30% of retinal ganglion cells have been lost; noticeable sight loss may take many years to develop. Vision loss due to glaucoma is described most often as the loss of peripheral vision. Patients may also complain about blurriness, dimness, or cloudiness. In patients with POAG, an ophthalmoscopic examination will show that the optic nerve takes a hollowed-out appearance, which is associated with loss of ganglion cell axons. There may be elevated IOP; however, there is no pain, redness, or visual symptoms, nor is there loss of visual acuity. With the progression of the disease, patients may experience central visual field loss, and in an untreated person, blindness can occur in 25 years.1-3,12-15
POAG is characterized by the narrowing or the closure of the anterior chamber angle. With this narrowing, there is improper drainage of aqueous humor fluid, leading to increased IOP. The speed and the degree of the increase in IOP determine whether the individual may experience symptoms. A quick rise in IOP, a typical characteristic of angle-closure glaucoma, can result in decreased vision, halos around lights, headache, severe eye pain, nausea, and vomiting. Signs that may suggest a rapid rise in IOP include conjunctival redness, mildly dilated pupil with poor reaction to light, and corneal swelling or cloudiness. If the rise in IOP is slower and does not reach high levels, the patient may be symptom-free.1-3,12-15
Management
The currently available medications for glaucoma all aim to lower the IOP and prevent further damage to the optic nerve. When caring for patients with glaucoma, the goals of therapy include lowering IOP, preventing further damage to the optic nerve, and preventing further visual function loss while increasing the patient’s quality of life with minimal adverse effects from treatment. Currently, there are several classes of medications that can be utilized for the management of POAG and chronic PACG. All medications either increase the aqueous outflow or decrease aqueous production (see TABLE 3). Preferred initial therapies include prostaglandins and beta-blockers, followed by the other available classes. Often, multiple medications may need to be administered to cause a greater reduction in IOP. Studies have shown that in patients using combination therapy from different classes, such as a beta-blocker with a prostaglandin, greater IOP reduction was seen.16,17
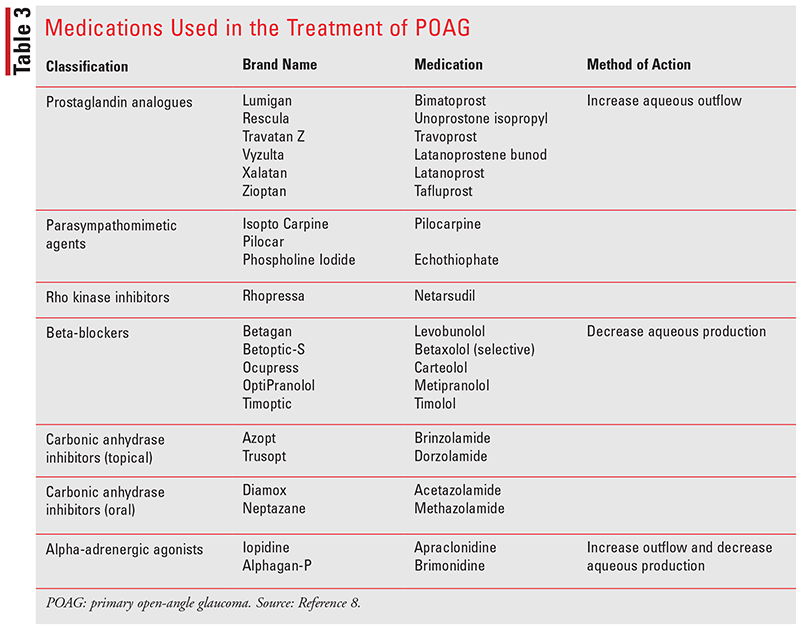
Prostaglandin analogues are considered first-line therapy. They effectively reduce IOP by up to 30% by improving uveoscleral outflow, lack systemic side effects, and require once-daily administration. Although systemic side effects are rare, patients may experience transient ocular effects, including blurred vision, itching, burning, or stinging and excessive tearing. Other common side effects are quite different from other ophthalmic agents and therefore require patient education. The prostaglandin analogues may lengthen eyelashes and cause hyperpigmentation of the iris (especially in those with light-colored eyes) and of lids and periorbital skin. The hyperpigmentation of the iris appears to be permanent, but eyelid and eyelash changes are usually reversible upon discontinuation. These agents should be used with caution in patients with active intraocular inflammation or in those with a history of intraocular inflammation.1,8,18-22
An alternative to the prostaglandin analogues is the ophthalmic beta-blockers. This class of medications reduces IOP by decreasing aqueous humor production. Common side effects include burning or stinging in the eyes, inflammation, and blurred vision. Although approved for twice-daily dosing, these agents are now more commonly dosed once daily; twice-daily dosing appears to provide no additional benefit compared with once daily in the morning and may likely increase the risk of systemic events. Unlike the prostaglandin analogues, this class of medication has the potential to cause systemic effects, especially in patients with pulmonary, cardiac, or metabolic disorders. Systemic effects include hypotension, bradycardia, and bronchospasms. Betaxolol, which is beta selective, may minimize the systemic effects but is associated with reduced lowering of IOP compared with the nonselective agents. Closing the eyes and practicing nasolacrimal occlusion following administration of the medication can help minimize systemic effects by limiting systemic absorption through the lacrimal ducts.1,8,22
Other medications that are used in the management of glaucoma include alpha-2 adrenergic agonists, topical and oral carbonic anhydrase inhibitors (CAIs), rho kinase inhibitors, and parasympathomimetics. Alpha-2 adrenergic agonists lower IOP by decreasing aqueous humor production and by increasing uveoscleral outflow. Although the efficacy of alpha-2 adrenergic agonists is similar to beta-blockers, their side effect profile limits their use. Allergic conjunctivitis is common with these agents and is a cause for discontinuation in many patients. Other common ocular side effects include stinging, conjunctival hyperemia, foreign-body sensation, and dry eyes. Systemic side effects include hypertension, dry mouth, fatigue, dizziness, and mental confusion.1,8,22
CAIs reduce aqueous humor production and are available as both oral and topical formulations. Oral CAIs are more effective compared to the topical formulations; however, their use is limited due to their significant systemic effects, including metabolic acidosis, paresthesia, bone marrow suppression, and renal calculi. Common side effects of topical agents include taste disturbances, blurred vision, and eye discomfort. Although CAIs are sulfonamide agents, topical agents have been well tolerated in most patients with sulfonamide allergies.1,8,22
Cholinergic agents lower IOP by increasing aqueous outflow through the trabecular meshwork. Once considered first-line agents, their use has declined over the years due to their unfavorable side effect profile and they are considered third line agents in the treatment of glaucoma. They cause contraction of the ciliary muscle, which leads to an increase of aqueous humor outflow. Local and systemic adverse effects are associated with their use. Locally they can cause miosis, which decreases vision acuity at night, a paradoxical increase in IOP, and retinal detachment. Systemic effects, although rare, include sweating, nausea, headache, increased salivation, and changes in blood pressure.1,8,22
Rho kinase inhibitors are the newest class of agents to be approved for the management of POAG. This class of medications decreases the resistance in the trabecular network, increasing outflow. Once considered first-line agents, their use has declined over the years due to their unfavorable side-effect profile and they are considered third-line agents in the treatment of glaucoma. They cause contraction of the ciliary muscle, which leads to an increase of aqueous humor outflow. Local and systemic adverse effects are associated with their use. Locally they can cause miosis, which decreases vision acuity at night, a paradoxical increase in IOP, and retinal detachment. Systemic effects, although rare, can include sweating, nausea, headache, increased salivation, and changes in blood pressure.1,8,22
Role of the Pharmacist
Pharmacists play a key role in assisting patients seeking treatment for glaucoma, as medications such as eye drops are often considered the first choice in treatment. The use of medications alongside laser treatment has proven to be a safe method to alleviate and control a patient’s eye pressure. However, while certain eye drops have proven to be beneficial in treating symptoms of glaucoma, they are only effective if taken as properly prescribed. Pharmacists can assist patients by providing education on proper use and offering knowledge on tools and techniques that can be used to ensure all patients receive the most beneficial outcomes, ensuring the best quality of life.6,23,24
One major obstacle that can impact patients’ results is their adherence to taking their medications. Patient adherence to glaucoma medication has been shown to be poor: Almost one-half of patients stop using their medication after 6 months. Nonadherence to glaucoma treatments can contribute significantly to irreversible blindness in many patients. Studies have identified a variety of barriers associated with poor adherence. These include low self-efficacy, forgetfulness, and difficulty with drop administration, especially in older patients. Pharmacists can use communication techniques to help determine if patients understand their disease state and how to properly use their medications. Pharmacists should discuss with patients their daily medication regimen and offer advice and tips that could potentially prevent a missed dose. Recommendations can include using the medications around the same time daily to build a routine, administering the medication with a regular daily activity such as brushing one’s teeth, or scheduling a daily reminder on a phone or alarm clock.6,23,24
Another common concern with glaucoma medications is difficulty applying eye drop medications. Many patients are uncomfortable administering eye drops or have improper application technique. This may result in not properly receiving the fully prescribed dose, which can impact efficacy. To assist these patients, pharmacists should provide demonstrations on proper eye drop administration techniques as well as recommend devices and tools such as eye drop applicators and dispensers, which assist in the application process.6,23,24
Patients may also have questions regarding the use of many OTC medications. Many OTC cold preparations’ drug labeling warns against their use if a patient has glaucoma. The key ingredients in most cold preparations include an antihistamine and a decongestant. Antihistamines and decongestants can induce pupillary mydriasis, which can increase IOP; however, this is more of a concern in patients with narrow-angle glaucoma. Most glaucoma patients are at very low risk of an increase in IOP, since almost 70% of patients are diagnosed with POAG.25
Pharmacists can instruct patients on how to properly administer eye drops and educate them about common side effects, as well as address questions pertaining to their treatment regimen. Pharmacists are the medication experts and can help patients see the importance of adherence to their medications by explaining the benefits of the treatment and help patients develop ways to remember to use their eye drops. In addition, they can remind them that if they are using multiple drops of the same medication or are instilling more than one medication, they should space the administration by at least 5 minutes. Overall, the impact a pharmacist can make on a patient’s life just by these small interventions can be life changing. These counseling points and educational tips can help patients get the treatment they need and prevent irreversible blindness.
Conclusion
Glaucoma is a very complex and dangerous disease that develops over time and, if left untreated, will lead to irreversible vision loss. There are medications that can prevent the progression of the disease, and pharmacists play a very important role in educating patients about these medications and the benefits of drug therapy. Patients may be wary of starting the treatment regimens, but counseling can help patients feel more comfortable. The counseling that pharmacists, as the most accessible healthcare provider, deliver is beneficial and will motivate the patients to be adherent to their medications. Glaucoma may have no cure, but it is treatable. Pharmacists can help with patient adherence and drug management to prevent the detrimental effects of glaucoma.

What Are the Symptoms of Glaucoma?
The symptoms can be so subtle that it is difficult to know you are developing the disease. It causes no pain, and your vision is normal in the beginning. Gradually you will start to lose your peripheral vision. Patients with known risk factors should be assessed by ophthalmologists regularly. Blurred vision, appearance of halos, pain, and redness of eyes are all signs of an acute glaucoma attack. If you experience this, call your eye doctor right away.
Who Is at Risk of Getting Glaucoma?
Individuals aged older than 60 years are at an increased risk of getting glaucoma. It is recommended that people in this age group receive regular eye exams. African Americans are more likely to develop the disease than Caucasians. Certain medical conditions, such as diabetes and hypertension, also put you at an increased risk of developing the disease. Trauma and physical injury to the eyes definitely can damage the optic nerve, resulting in glaucoma. Corticosteroid use for prolonged duration puts patients at an increased risk of getting glaucoma.
How Is Glaucoma Diagnosed?
An eye doctor determines if an individual has glaucoma through a dilated eye exam. During the exam, the doctor is able to assess the inside of the eye, in addition to measuring the eye pressure.
Do I Need to Be Screened for Glaucoma if I Do Not Have Any of the Symptoms?
It is recommended to be screened if you do have certain risk factors. Some of these risk factors include being over 40 years of age, having a family history of glaucoma, having diabetes, having high blood pressure, and being of African or Hispanic descent. The exams are not dangerous, and diagnosing the disease early on would help in early treatment and preventing it from having a big impact on your vision.
Can Glaucoma Be Treated?
Although there is no cure, there are many options to help manage glaucoma. The most common and often first treatment option is using medicated eye drops to lower the pressure in your eye. This will help prevent nerve damage and stop vision loss. It is important to remember to use the eye drops every day. Some cases may require laser treatment or surgery. Talk with your eye doctor about the best treatment for you.
Where Can I Go for More Information?
You can visit the following websites for additional support and resources:
National Institutes of Health, National Eye Institute: www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/glaucoma
American Academy of Ophthalmology: www.aao.org/eye-health/diseases/what-is-glaucoma
REFERENCES
1. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma. JAMA. 2014;311(18):1901-1911.2. Jonas JB, Aung T, Bourne RR, et al. Glaucoma. Lancet. 2017;390(10108):2183-2193.
3. Greco A, Rizzo MI, De Virgilio A, Gallo A, et al. Emerging concepts in glaucoma and review of the literature. Am J Med. 2016;129(9):1000.e7-1000.e13.4. Allison K, Patel D, Alabi O. Epidemiology of glaucoma: the past, present, and predictions for the future. Cureus. 2020;12(11):e11686.
5. BrightFocus Foundation. Glaucoma: facts & figures. July 6, 2015. www.brightfocus.org/glaucoma/article/glaucoma-facts-figures. Accessed December 8, 2020.6. Dreer LE, Girkin C, Mansberger SL. Determinants of medication adherence to topical glaucoma therapy. J Glaucoma. 2012;21(4):234-240.
7. McMonnies CW. Glaucoma history and risk factors. J Optom. 2017;10(2):71-78.8. Gedde SJ, Vinod K, Wright MM, et al. Primary open-angle glaucoma preferred practice pattern. Ophthalmology. 2021;128(1):P71-P150.
9. Gedde SJ, Chen PP, Muir KW, et al. Primary angle-closure disease preferred practice pattern. Ophthalmology. 2021;128(1):P30-P70.10. Friedman DS, Wolfs RCW, O’Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4):532-538.
11. Zhao D, Cho J, Kim MH, et al. Diabetes, fasting glucose, and the risk of glaucoma: a meta-analysis. Ophthalmology. 2015;122(1):72-78.12. Gupta D, Chen PP. Glaucoma. Am Fam Physician. 2016;93(8):668-674.
13. Heijl A, Bengtsson B, Hyman L, et al. Natural history of open-angle glaucoma. Ophthalmology. 2009;116(12):2271-2276.14. Cohen LP, Pasquale LR. Clinical characteristics and current treatment of glaucoma. Cold Spring Harb Perspect Med. 2014;4(6):a017236.
15. Hu CX, Zangalli C, Hsieh M, et al. What do patients with glaucoma see? Visual symptoms reported by patients with glaucoma. Am J Med Sci. 2014;348(5):403-409.16. Stein JD, Khawaja AP, Weizer JS. Glaucoma in adults—screening, diagnosis, and management: a review. JAMA. 2021;325(2):164-174.
17. Lindén C, Heijl A, Jóhannesson G, et al. Initial intraocular pressure reduction by mono- versus multi-therapy in patients with open-angle glaucoma: results from the Glaucoma Intensive Treatment Study. Acta Ophthalmol. 2018;96(6):567-572.18. Xalatan (latanoprost) package insert. New York, NY: Pfizer, Inc; 2022.
19. Travatan Z (travoprost) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2022.20. Lumigan (bimatoprost) package insert. Madison, NJ: Allergan USA, Inc; 2022.
21. Zioptan (tafluprost) package insert. Lake Forest, IL: Akorn, Inc: 2022.22. Marquis RE, Whitson JT. Management of glaucoma: focus on pharmacological therapy. Drugs Aging. 2005;22(1):1-21.
23. Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122(7):1308-1316.24. Feehan M, Munger MA, Cooper DK, et al. Adherence to glaucoma medications over 12 months in two US community pharmacy chains. J Clin Med. 2016;5(9):79.
25. Kabat AG, Sowka J. When OTC is not OK. December 15, 2008. www.reviewofoptometry.com/article/when-otc-is-not-ok. Accessed December 5, 2022.26. Chuck RS, Dunn SP, Flaxel CJ, et al. Comprehensive adult medical eye evaluation preferred practice pattern. Ophthalmology. 2021;128(1):P1-P29.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





