US Pharm. 2022;47(10):6-9.
ABSTRACT: Hypertensive disorders in pregnancy affect 15 out of 100 pregnancies within the United States, placing both the mother and fetus at risk for short- and long-term complications. As more women enter pregnancy at an older age, are obese, or with preexisting health conditions, this number will likely continue to rise. Pharmacists are perfectly positioned to intervene by providing education to patients on risk factors, mitigation strategies, and medication selection.
High arterial blood pressure (BP), or hypertension (HTN), is a condition that afflicts about 50% of adults in the United States.1 While this rate has remained relatively consistent over the past 20 years, the most recent data from the CDC shows growth in the number of women with hypertensive disorders in pregnancy (HDP).2 In 2019, the prevalence of HDP among delivery hospitalizations in the U.S. was 15.9%, an increase of 2.6% from 2017. African American and non-Hispanic American Indian/Alaska Native women are disproportionally affected, as well as those residing in the lowest median household income quartile or in the U.S. Midwest and South. Additionally, research indicates that the increase in HDP prevalence is not isolated to the U.S. but is a worldwide issue in developed countries. Modifiable risk factors worldwide contributing to this growth include an increase in maternal age and preexisting maternal comorbidities (e.g., HTN, diabetes, chronic kidney disease [CKD], obesity).3
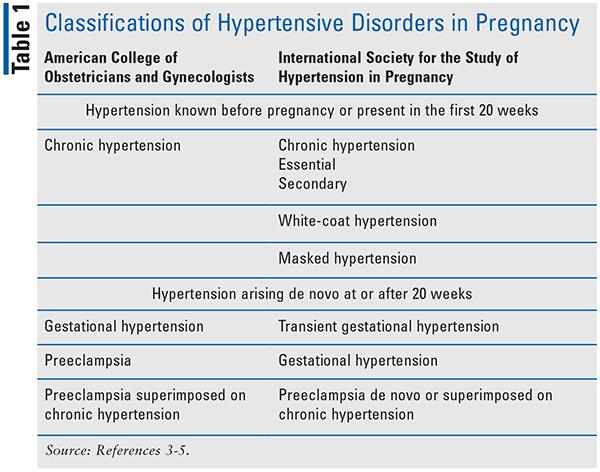
Classifications of HDP are broadly based on gestational age at the time of HTN diagnosis and presence of proteinuria.3-5 The American College of Obstetricians and Gynecologists (ACOG) classifies HDP into four categories, whereas the International Society for the Study of Hypertension in Pregnancy (ISSHP) lists six main classifications (see TABLE 1). Contrary to the current American College of Cardiology and American Heart Association diagnostic criteria for HTN in nonpregnant patients, ACOG and ISSHP define HTN as a systolic BP of 140 mmHg or higher or a diastolic BP of 90 mmHg or higher, or both. HDP is linked to increased risk for maternal mortality and contributes to short- and long-term maternal and fetal/offspring morbidity. Maternal short-term complications may include myocardial infarction (MI), stroke, cardiomyopathy, spontaneous coronary artery dissection, and postpartum hemorrhage; long-term, women are at higher risk for development of cardiovascular disease (HTN, dyslipidemia, MI, heart failure, atrial fibrillation, stroke), diabetes, and CKD. Fetal short-term complications may include neonates that are small for gestational age, stillbirth, and preterm delivery, which may then lead to long-term complications.
Chronic HTN
The diagnosis of chronic HTN encompasses women who enter pregnancy with a prior history of HTN, development of HTN before 20 weeks’ gestation, or HTN persisting beyond 12 weeks postpartum.6 The current discrepancy in BP cutoffs used to define HTN for nonpregnant women, ≥130/80 mmHg, compared with women during pregnancy, ≥140/90 mmHg, has created uncertainties in BP control and treatment for women diagnosed with HTN prior to pregnancy. Estimates released in 2019, which defined HTN as a BP ≥140/90 mmHg, placed the prevalence of HTN in nonpregnant women of reproductive age at 9.3%.7 During pregnancy, systemic vascular resistance decreases in response to a physiologic increase in plasma volume and reciprocally cardiac output.8 For most women, this correlates to a decrease in BP about the middle of their first trimester that nadirs around 16 to 20 weeks’ gestation. The BP decrease may be as significant as 10%, making it challenging to detect all women with chronic HTN if prepregnancy BPs are unknown.
There are two general classification schemes for chronic HTN: one based on cause and the other on degree of BP elevation.6 Similar to nonpregnant hypertensive patients, most women diagnosed with chronic HTN in pregnancy have essential HTN, or elevation due to unknown case, with only 11% to 14% of patients having secondary HTN due to underlying renal, endocrine, or vascular conditions. Additionally, chronic HTN may be classified as mild, a BP of 140-159 mmHg/90-109 mmHg, or severe if BP elevations reach 160/110 mmHg or higher. Patients must have two clinic BP readings, at least 4 hours apart, that are consistently elevated in order to meet the diagnosis for HTN. Home BP monitoring may be considered to rule out white coat HTN or masked HTN.
Given that chronic HTN places the mother and fetus at risk for complications during and after pregnancy, care must be taken to properly screen, closely monitor, and treat these women.6 The most recent clinical management guidelines published by ACOG on management of chronic HTN in pregnancy (2018) provide conservative recommendations, which note a number of ongoing trials that could alter clinical practice. Self-monitoring of BP or home monitoring was one such area. The BUMP1 randomized clinical trial assessed self-monitoring of BP with telemonitoring compared with monitoring solely at antenatal appointments (standard of care) in women at higher risk for preeclampsia.9 The trial failed to show a statistically significant difference in time to detection of HTN between the two cohorts. The BUMP2 randomized clinical trial enrolled patients with chronic or gestational HTN to assess the role of self-monitoring on BP control.10 Results showed no statistically significant difference in BP control for women who conducted self-monitoring of BP as opposed to those who underwent BP monitoring solely at antenatal appointments. While self-monitoring may be helpful for patients who have difficulty making antenatal appointments, these trials suggest no clear benefit to all patients, particularly those being closely monitored by a provider.
Once a diagnosis of HTN is reached, whether it be chronic or gestational, the question shifts to management of the condition, weighing the risk of undertreatment to those of pharmacologic agents. Historically, the guidelines favored a conservative approach to treatment initiation, placing the BP threshold for initiation or titration of antihypertensives in pregnancy at 160/110 mmHg.6 In April 2022, the results of the Chronic Hypertension and Pregnancy (CHAP) study were published.11 This multisite clinical trial enrolled women diagnosed with HTN prior to 23 weeks’ gestation and randomized them to receive antihypertensive therapy at a threshold of 140/90 mmHg (active) or 160/110 mmHg (control) to evaluate the benefit and safety of mild HTN treatment. Women in the active treatment arm saw better pregnancy outcomes (reduction in preeclampsia with severe features, medically indicated preterm birth, placental abruption, or fetal/neonatal death) without an increase in the incidence of small-for-gestational-age birthweight. ACOG subsequently released a practice advisory decreasing the BP threshold for initiation or titration of antihypertensives in pregnancy to 140/90 mmHg, noting that trials are still needed to establish a goal BP.12 Currently, the recommendation for pregnant women with chronic HTN receiving pharmacologic treatment is to maintain BP between 120/80 mmHg and 160/110 mmHg.6 For women who enter into pregnancy with a diagnosis of HTN and are receiving pharmacologic therapy, the guidelines support continuation of that agent provided that the medication poses no risk to the fetus.
Gestational HTN and Preeclampsia
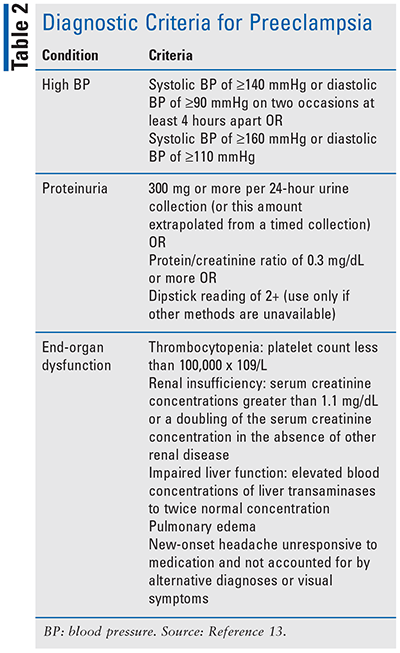
Gestational HTN is defined as high BP that develops after 20 weeks’ of gestation.13 If this is not well controlled or the mother has other risk factors, HTN can lead to a serious condition called preeclampsia. Preeclampsia is high BP after 20 weeks’ gestation with end-organ dysfunction (see TABLE 2), which places the mother at an increased risk for seizures, coma, and/or death. There are also elevated rates of fetal morbidity and mortality due to increased risk of preterm labor, growth restriction, and a variety of other complications. Early identification of women at increased risk for developing HTN and preeclampsia is imperative, as prophylactic measures can be taken to minimize complications to the mother and fetus.13-15 Direct risk factors include chronic HTN, history of preeclampsia in a previous pregnancy, or family history of preeclampsia. Additional factors, such as obstructive sleep apnea, nulliparity, multifetal gestations, obesity, older maternal age, thrombophilia, diabetes, etc., can compromise organ function and indirectly affect the mother’s risk.
Risk levels for developing preeclampsia can be decreased by change in diet and physical activity.14,16 Dietary recommendations include limiting salt intake, drinking six to eight glasses of water a day, avoiding fried/processed foods, and avoiding caffeine. Patients are encouraged to exercise regularly; however, they should get an adequate amount of rest and keep their feet elevated several times a day. The U.S. Preventive Services Task Force recommends that moderate- to high-risk patients take low-dose aspirin after 12 weeks’ gestation.13,17,18 Metformin, sildenafil, and statins have been investigated for prophylactic therapy but have not been recommended for use outside of clinical trials.
Management of HTN and Preeclampsia
Management of HTN in pregnancy is the same regardless of the time when it develops.19,20 The goal is to maximize nonpharmacologic options, for example, DASH diet, sodium restriction, and exercise, with pharmacologic agents added as needed. Management of preeclampsia is also a combination of lifestyle modifications and antihypertensive medications. The drug of choice will be based on the patient’s medical history, the agent’s side-effect profile, severity, and agent availability. These agents include methyldopa, labetalol, nifedipine, nicardipine, esmolol, hydralazine, and nitroglycerin.17,18
Methyldopa: Methyldopa works by binding alpha-2 adrenergic receptors and reducing total peripheral vascular resistance.21 This would be contraindicated if the patient has an active hepatic disease or is taking a monoamine oxidase inhibitor. This should not be used for postpartum HTN due to risk of postpartum depression. Methyldopa may also cause edema, reversible granulocytopenia, reversible thrombocytopenia, hemolytic anemia, hepatic disorders, and/or sedation. This can be used for nonsevere preeclampsia but is not preferred due to adverse effects.19
Labetalol (Off-Label Use): Labetalol blocks alpha- and beta-adrenergic receptors, which decreases vascular resistance and slows heart rate.22 Labetalol may cause hepatic injury or hypotension with or without syncope. Newborns exposed to labetalol should be monitored for 48 hours for bradycardia, hypoglycemia, and respiratory depression. Labetalol should not be used in patients with asthma, heart failure, greater than first-degree heart block, cardiogenic shock, severe bradycardia, or other conditions associated with severe hypotension. This is primarily used for acute severe preeclampsia in IV formulations but can also be given orally for nonsevere preeclampsia.19,20
Nifedipine (Off-Label Use): Nifedipine is an oral L-type calcium channel blocker and decreases vascular resistance. It is available orally as extended-release and immediate-release formulations.23 The extended-release formulation is preferred due to the hypotension/syncope risk. This is used for acute-onset, severe HTN in preeclampsia and eclampsia as well as nonsevere preeclampsia.19,20
Nicardipine: Nicardipine is also a calcium channel blocker. It is given orally for HTN in pregnancy but is also given as a rapid-acting, continuous IV infusion for severe preeclampsia and eclampsia.19,20 The IV formulation has no renal or hepatic adjustments.24
Esmolol (Off-Label Use): Esmolol is a selective beta-1 blocker. It is used off-label for hypertensive emergencies, including severe preeclampsia and eclampsia.20 It is given as an IV infusion. There are no renal or hepatic adjustments. This is contraindicated for patients with severe sinus bradycardia, heart block greater than first degree, sick sinus syndrome, decompensated heart failure, cardiogenic shock, and pulmonary HTN. Use of esmolol in pregnancy has a risk of fetal bradycardia, growth restriction, hypoglycemia, and respiratory depression. The newborn should be monitored for at least 48 hours after delivery.25
Hydralazine (Off-Label Use): Hydralazine is a vasodilator. It is given orally for hypertensive emergencies in pregnancy or postpartum.20 There are renal impairment considerations when scheduling doses, but no hepatic adjustments. It is contraindicated in patients with coronary artery disease and mitral valve rheumatic heart disease. Females are at risk for developing hydralazine-induced lupus-like syndrome after at least 3 months of therapy and should be monitored closely post therapy.26
Nitroglycerin: IV nitroglycerin is used for both hypertensive emergency and uterine relaxation.20 Because it is also used adjunctively in acute decompensated heart failure patients to reduce volume overload, this medication is preferred for severe preeclampsia treatment if the patient has pulmonary edema. There are no renal or hepatic adjustments. IV nitroglycerin is contraindicated in patients with increased intracranial pressure.27
Labor Induction: The current recommendations from ACOG Bulletin 222 state that induction of labor should be considered for all pregnant women beyond 34 weeks’ gestation with severe hypertensive disease and beyond 37 weeks with mild hypertensive disease. Labor induction before this point is associated with higher neonatal intensive care unit admission rates, neonatal respiratory complications, and neonatal death.13 However, there are maternal risks associated with expectant management, such as development of hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome, eclampsia, new-onset severe preeclampsia, thromboembolic disease, pulmonary edema, postpartum hemorrhage, and placental abruption.13, 28 In summary, induction of labor is associated with maternal benefit, while expectant management is intended for neonatal benefit. Risks and benefits should be assessed for each patient. Certain conditions of the mother and/or fetus preclude expectant management, including uncontrolled severe HTN resistant to antihypertensive medications, HELLP syndrome, eclampsia, myocardial infarction, persistent headaches or epigastric pain resistant to treatment, pulmonary edema, abnormal fetal testing, fetal death, etc.13
Eclampsia
Eclampsia is defined as new-onset seizures or coma (in the absence of causative conditions such as epilepsy or stroke) in a patient with preeclampsia or gestational HTN and can occur before, during, or after labor.13 It is usually preceded by a prodromal phase consisting of persistent headaches, blurred vision, photophobia, and altered mental status, but it may occur with no warning signs. It may also occur in women who have no classic signs or prior diagnosis of preeclampsia. It is important to note that only a small proportion of women with preeclampsia will develop eclamptic seizures without prophylaxis and that progression to eclampsia from preeclampsia is not linear.
Management of Eclampsia
Magnesium Sulfate: Magnesium sulfate is associated with improved fetal and maternal morbidity and is superior to phenytoin, diazepam, and nimodipine for reducing eclamptic seizures. Because of this, the ACOG Practice Bulletin 222 lists it as the preferred choice for the treatment and prevention of initial and recurrent eclamptic seizures.13,17 The recommended dosing regimen for magnesium sulfate for eclampsia is a loading dose of 4 to 6 g IV infused over 15 to 20 minutes, followed by a 2-g/hour maintenance infusion.17 In the event of a seizure during infusion, a 2-g bolus may be given, but the maximum daily dosage should not exceed 8 g. Benzodiazepines and phenytoin should only be used in the context of epileptic treatment or when magnesium sulfate is unavailable or contraindicated.13 Some situations in which magnesium sulfate would be contraindicated include myasthenia gravis, hypocalcemia, moderate-to-severe renal failure, cardiac ischemia, heart block, or myocarditis. Labor should never be induced prior to maternal stabilization.13
Seizure Management
For an important reminder regarding the management of a seizure, see TABLE 3.29 During an eclamptic seizure, the fetus may develop hypoxia-related bradycardia but usually recovers.17
HELLP Syndrome
HELLP syndrome is a rare complication that is commonly misdiagnosed because it presents with very general symptoms: malaise, epigastric pain, nausea and vomiting, and headache.30 The pathogenesis is not well understood, and no common precipitating factor has been found. Labor induction is indicated at 34 weeks’ gestation and beyond if HELLP is diagnosed or if maternal or fetal condition worsens. Prior to this point, expectant management includes corticosteroid and magnesium sulfate administration. Expectant mothers who are diagnosed with HELLP syndrome are more likely to experience recurrence in later pregnancies or develop preeclampsia and eclampsia.30,31 Infant morbidity and mortality rates range from 10% to 60%, while the mother may develop serious complications, such as disseminated intravascular coagulation, placental abruption, adult respiratory distress syndrome, hepatic and renal failure, pulmonary edema, and hepatic rupture.30
Conclusion
As pharmacists, one of our most important roles is education, whether that be providers or patients. In the hospital setting, our goal is to aid in prompt BP reduction without placing the mother or fetus at risk and through fast identification of patients at risk for developing eclampsia. In the community, BP screenings can help to identify women with HTN who are at risk for preeclampsia. Additionally, women of childbearing age should be educated on the risks associated with the development and progression of HTN, particularly the effects it can have on reproduction.
REFERENCES
1. Ostechega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension prevalence among adults aged 18 and over: United States, 2017-2018. NCHS Data Brief. 2020;(364):1-8.
2. Ford ND, Cox S, Ko JY, et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization—United States, 2017-2019. MMWR Morb Mortal Wkly Rep. 2022;71:585-591.
3. Garovic VD, Dechend R, Easterling T, et al. Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: a scientific statement from the American Heart Association [published correction appears in Hypertension. 2022;79(3):e70]. Hypertension. 2022;79(2):e21-e41.
4. Ying W, Catov JM, Ouyang P. Hypertensive disorders of pregnancy and future maternal cardiovascular risk. J Am Heart Assoc. 2018;7(17):e009382.
5. Brown MA, Magee LA, Kenny LC, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72(1):24-43.
6. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletin—Obstetrics. ACOG Practice Bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133(1):e26-e50.
7. Azeez O, Kulkarni A, Kuklina EV, et al. Hypertension and diabetes in non-pregnant women of reproductive age in the United States. Prev Chronic Dis. 2019;16:E146.
8. Chandrasekaran S, Badell ML, Jamieson DJ. Management of chronic hypertension during pregnancy. JAMA. 2022;327(17):1700-1701.
9. Tucker KL, Mort S, Yu L, et al. Effect of self-monitoring of blood pressure on diagnosis of hypertension during higher-risk pregnancy: the BUMP 1 randomized clinical trial. JAMA. 2022;327(17):1656-1665.
10. Chappell LC, Tucker KL, Galal U, et al. Effect of self-monitoring of blood pressure on blood pressure control in pregnant individuals with chronic or gestational hypertension: the BUMP 2 randomized clinical trial. JAMA. 2022;327(17):1666-1678.
11. Tita AT, Szychowski JM, Boggess K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386(19):1781-1792.
12. American College of Obstetricians and Gynecologists. Clinical guidance for the integration of the findings of the chronic hypertension and pregnancy (CHAP) study. April 2022. www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2022/04/clinical-guidance-for-the-integration-of-the-findings-of-the-chronic-hypertension-and-pregnancy-chap-study. Accessed June 28, 2022.
13. Gestational hypertension and preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135(6):e237-e260.
14. American Pregnancy Association. Preeclampsia. December 9, 2021. https://americanpregnancy.org/healthy-pregnancy/pregnancy-complications/preeclampsia/. Accessed June 27, 2022.
15. ACOG Committee Opinion No. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132(1):e44-e52.
16. WHO recommendation on calcium supplementation before pregnancy for the prevention of pre-eclampsia and its complications. Geneva, Switzerland: World Health Organization; 2020.
17. Leeman L, Dresang LT, Fontaine P. Hypertensive disorders of pregnancy. Am Fam Physician. 2016;93(2):121-127.
18. U.S. Preventive Services Taskforce. Preeclampsia: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/preeclampsia-screening#fullrecommendationstart. Accessed August 12, 2022.
19. WHO recommendations on drug treatment for non-severe hypertension in pregnancy. Geneva: World Health Organization; 2020.
20. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75(6):1334-1357.
21. Aldomet (methyldopa) package insert. Whitehouse Station, NJ: Merck & Co., Inc; 1962.
22. Trandate (labetalol hydrochloride) package insert. San Diego, CA: Prometheus Laboratories Inc; 2010.
23. Procardia (nifedipine) package insert. New York, NY: Pfizer; 1981.
24. Cardene (nicardipine hydrochloride) package insert. Bedminster, NJ: EKR Therapeutics; 1988.
25. Brevibloc (esmolol hydrochloride) package insert. Deerfield, IL: Baxter Healthcare; 1986.
26. Hydralazine hydrochloride package insert. Shirley, NY: American Regent Inc; 1997.
27. Nitroglycerin in 5% dextrose injection package insert. Mississauga, ON: Baxter Healthcare; 1989.
28. Koopmans CM, Bijlenga D, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374(9694):979-988.
29. CDC. Seizure first aid. January 2, 2022. www.cdc.gov/epilepsy/about/first-aid.htm. Accessed August 8, 2022.
30. Padden MO. HELLP syndrome: recognition and perinatal management. Am Fam Physician. 1999;60(3):829-836.
31. Kınay T, Küçük C, Kayıkçıoğlu F, Karakaya J. Severe preeclampsia versus HELLP syndrome: maternal and perinatal outcomes at <34 and ≥34 weeks’ gestation. Balkan Med J. 2015;32(4):359-363.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






