US Pharm. 2016;41(5):HS8-HS12.
ABSTRACT: Hypertrophic cardiomyopathy is a common heart disorder, usually genetic in origin, that may affect up to 600,000 people in the United States. The disorder, which is characterized by left ventricular hypertrophy, is usually not progressive, but a small subset of patients develop serious complications, such as progressive heart failure, atrial fibrillation, and sudden cardiac death. Pharmacologic treatment of symptomatic hypertrophic cardiomyopathy includes beta-blockers and nondihydropyridine calcium channel blockers. Treatment monitoring and medication education should be included as part of a global treatment approach. Pharmacists play an important role in educating patients on the importance of screening and of adhering to lifestyle and treatment recommendations.
Hypertrophic cardiomyopathy (HCM) is a cardiac contractile-muscle disorder most often caused by genetic mutations. HCM is a global disease that is characterized by phenotypically positive left ventricular hypertrophy (LVH). It occurs at a rate of one in 500 (0.2%) in the general population, which translates to approximately 600,000 people affected in the United States—however, many individuals go unidentified.1 LVH in HCM is not progressive in the vast majority, having an annual mortality rate of 1%.1 However, a small subset of patients develop serious complications, including progressive heart failure (HF) associated with systolic dysfunction, atrial fibrillation (AF) with increased stroke risk, and sudden cardiac death (SCD).2,3 HCM is characterized by LVH, deranged cardiomyocyte energetics, diastolic dysfunction, microvascular ischemia, enhanced myocardial fibrosis, abnormal sympathetic innervation, multifactorial arrhythmogenesis, and dynamic left ventricular outflow tract (LVOT) obstruction.4-6 LVOT obstruction is a major determinant of symptoms (i.e., dyspnea, chest pain, andpresyncope) and represents a major therapeutic target.
Pathophysiology
Autosomal-dominant gene mutations of the sarcomere and its constituent myofilament elements are thought to be the cause of cardiac hypertrophy.7-10 Consequently, various signaling pathways and disease mechanisms can be activated, including disturbed biomechanical stress sensing, impaired calcium cycling and sensitivity, altered energy homeostasis, and increased fibrosis. In terms of physiopathology, sarcomere mutations result in cardiac hypertrophy, interstitial fibrosis, and coronary microvascular remodeling. These changes lead to increased extravascular compression forces, reduced capillary density, and microvascular dysfunction. Under the influence of neurohormonal triggers, HCM patients develop myocardial ischemia and LV remodeling and fibrosis.11 The clinical consequences of HCM include LVOT obstruction, myocardial ischemia, diastolic dysfunction, and arrhythmias.12-14
LVOT Obstruction: LVOT obstruction encompasses a series of stenotic lesions that extend from the anatomical LVOT to the descending portion of the aortic arch. These lesions impose an increased afterload on the LV, later resulting in LVH, LV dilation, and LV failure. During systole, intraventricular pressure normally rises to the level of the aortic pressure, allowing the aortic valve to open and permitting the expulsion of blood from the LV.15 In HCM, a dynamic pressure gradient occurs wherein the LV pressure is higher than the aortic pressure. This gradient represents the degree of obstruction that must be overcome to allow for LV emptying. One-third of patients present with nonobstructive HCM (<30 mmHg gradient); the remaining two-thirds present with dynamic LVOT obstruction either at rest or upon physiological provocation (≥30 mmHg gradient).16-18
Myocardial Ischemia: Myocardial ischemia results from oxygen supply-and-demand mismatch. An increased oxygen demand and compromised coronary blood flow are caused by the thickened myocardium. Autonomic dysfunction, which is associated with SCD, often presents with an abnormal blood pressure response to exercise, with either a drop in blood pressure or failure of blood pressure increases >20 mmHg. These abnormalities result from obstruction or systemic vasodilation during exercise.19-22
Diastolic Dysfunction: Diastolic dysfunction arises from outflow obstruction, variable ventricular contraction and relaxation, and abnormal calcium reuptake resulting in delayed inactivation. Diastolic dysfunction affects ventricular relaxation and LV-chamber stiffness in the presence of normal LV systolic function—a key feature of HCM.23 Mitral regurgitation, which may also contribute to diastolic dysfunction, is caused by distortion of the mitral-valve apparatus induced by the systolic atrial motion.24
Atrial Fibrillation: AF often predicts poor outcomes, including HCM-related mortality, symptomatic deterioration, and risk of stroke. The condition may be more likely in HCM because of stiff ventricles and the loss of atrial transport.23
Clinical Presentation and Diagnosis
Many patients with HCM are asymptomatic, whereas others develop HF symptoms, including LVOT gradients, dyspnea on exertion, fatigue, atypical or anginal chest pain, presyncope, syncope, and palpitations. Advanced HF symptoms such as orthopnea, paroxysmal nocturnal dyspnea, and edema are uncommon. In the absence of symptoms, affected individuals are diagnosed as a result of family screening, detection of a murmur upon physical examination, or ECG abnormality.2,23
Suspicion of HCM is based on the presence of one or more of the following clinical findings: family history of HCM, unexplained cardiac symptoms (i.e., dyspnea, chest pain, fatigue, palpitations), systolic ejection murmur, and abnormal 12-lead ECG (i.e., LV hypertrophy or deep, broad Q waves) or syncopal episodes. The presence of any of these clinical findings should prompt further testing via serial ECG or 24-hour ambulatory ECG monitoring (Holter), Doppler studies, transthoracic echocardiography (TTE), cardiac MRI, and/or stress testing. Based on the presence of the key clinical findings, Doppler studies and TTE are performed to detect changes in the septal wall (septal-wall thickness ≥15 mm identifies HCM). Serial 12-lead ECG and/or 24-hour ambulatory ECG monitoring (Holter) is used to detect ventricular tachycardia (VT) and the possible need for implantable cardioverter defibrillator (ICD) therapy. A treadmill exercise test is used to determine the functional capacity and response to therapy and can be a useful tool to help stratify the risk of SCD. Treadmill tests should be reserved for patients with a peak resting gradient of <50 mmHg, which is considered severe and predicts a poor prognosis if HCM is left untreated.25-28
Genetic testing may also confirm the diagnosis in symptomatic patients. Commercially available genetic testing provides a definitive diagnosis and can assist early screening and detection of disease within families. Testing assesses the presence of eight genes known to definitively cause HCM, including beta-myosin heavy chain, myosin-binding protein C, troponin T, troponin I, alpha-tropomyosin, actin, regulatory light chain, and essential light chain.7-10,25,26,29 Evaluation of familial inheritance and genetic counseling are recommended as part of the assessment of patients with HCM. Some patients may have gene mutation without cardiac hypertrophy (genotype-positive/phenotype-negative). Serial ECG, TTE, and clinical assessment should be performed in genotype-positive/phenotype-negative patients.25,26
Risk Stratification and Prevention of SCD
Risk stratification of SCD in patients with HCM is useful in determining the need for ICD placement for primary prevention and should be performed every 1 to 2 years. Risk factors for SCD include 1) a personal history of ventricular fibrillation, sustained VT, or SCD events, including appropriate ICD therapy for ventricular tachyarrhythmias; 2) a family history of SCD events, including appropriate ICD therapy for ventricular tachyarrhythmias; 3) unexplained syncope; 4) documented nonsustained VT (≥3 beats at ≥120 bpm Holter); and 5) LV hypertrophy (maximal LV wall thickness ≥30 mm). The presence of any of these risk factors is an indication for ICD evaluation and placement.26
Treatment
Most patients with HCM will achieve a normal life expectancy. Patients and their families should receive education on screening recommendations for first-degree relatives and the avoidance of strenuous physical activity. Coronary artery disease is a common comorbidity; therefore, it is essential to provide aggressive risk modification by managing cardiovascular risk factors—including hypertension, diabetes, obesity, and hyperlipidemia—according to their respective guidelines. Low-intensity aerobic exercise and good hydration are also reasonable recommendations since many HCM patients have diastolic dysfunction and require relatively high filling pressure to achieve adequate ventricular filling. Patients should be educated on the avoidance of certain medications (i.e., high-dose diuretics, vasodilators, inotropes) that may cause vasodilation, thereby exacerbating the degree of obstruction.26
In symptomatic HCM, goals of therapy include alleviating exertional dyspnea, palpitations, and chest discomfort. Beta-blockers and nondihydropyridine (non-DHP) calcium channel blockers are the mainstay of therapy. Adjunctive therapies include IV phenylephrine, disopyramide, and diuretics. Anticoagulation and amiodarone are treatment options for patients who develop AF. Digitalis is not recommended for the treatment of dyspnea in the absence of AF. Patients who develop systolic dysfunction with an LV ejection fraction <50% should be treated in accordance with HF guidelines. Nonpharmacologic invasive treatments—septal reduction therapy (myectomy), alcohol septal ablation, pacemaker or ICD implantation, and heart transplant—are recommended when symptoms are refractory to drug therapies. The following section will discuss pharmacologic treatment options.26 TABLE 1 summarizes the recommendations of the American College of Cardiology Foundation/American Heart Association’s 2011 Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy.
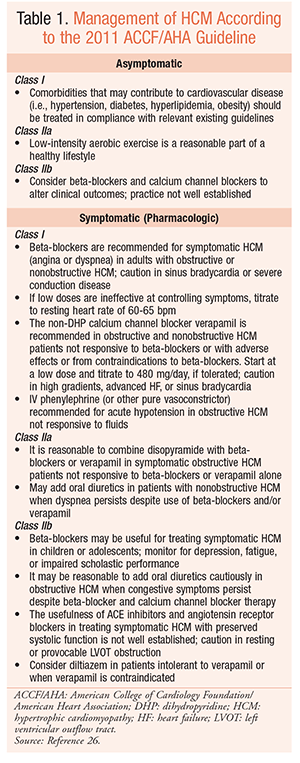
Beta-Blockers: Beta-blockers are recommended as first-line drug therapy in both obstructive and nonobstructive symptomatic HCM. Beta-blockers also may be used in children and adolescents with symptomatic HCM. The negative inotropic and chronotropic effects of beta-blockers attenuate adrenergic-induced tachycardia, ventricular contractility, and stiffness, thereby improving ventricular relaxation, increasing time for diastolic filling, and reducing excitability. Additionally, beta-blockers help restore the balance of supply-demand relationships and improve diastolic filling, which is facilitated by a more efficient inactivation of myocardial contractile proteins.26-28
The beta-blockers studied thus far include propranolol, nadolol, sotalol, and bisoprolol. Benefits of beta-blockers in reducing LVOT gradient and providing symptom relief were established in the late 1960s in small prospective studies evaluating propranolol’s therapeutic effect at doses of 80 to 300 mg per day.30-34 In a small double-blind, placebo-controlled trial comparing nadolol (80-160 mg daily) with verapamil (240-480 mg daily), nadolol was more effective at providing symptom relief in HCM.35 In a small placebo-controlled, double-blind crossover trial, sotalol 160 to 320 mg daily was effective at suppressing supraventricular tachycardia and ventricular arrhythmias.36 More recently, bisoprolol (3.5-10 mg daily) demonstrated marked reduction or elimination of exercise-induced LVOT obstruction.37
The beta-blocker dose should be titrated to a resting heart rate of <60-65 beats per minute or the maximum tolerated. No studies to date have determined which beta-blockers would provide the most benefit. Beta-blockers should be used in caution in patients with sinus bradycardia or severe conduction disease. Patients treated with beta-blockers should be monitored for depression, fatigue, and impaired work or school performance.26-28
Calcium Channel Blockers: Non-DHP calcium channel blockers are recommended in both obstructive and nonobstructive symptomatic HCM patients who do not respond to beta-blockers or who have adverse effects from or contraindications to beta-blockers. As with beta-blockers, calcium channel blockers’ negative inotropic effects are beneficial in HCM.26-28
Although other non-DHP calcium channel blockers may be used, verapamil is preferred. The effectiveness of verapamil (360-480 mg daily) has been demonstrated in studies, and the agent has been established as an alternative for patients intolerant to beta-blockers.38-45 In several small prospective studies, diltiazem titrated to 360 mg has been shown to improve diastolic function and myocardial ischemia.46-49
Calcium channel blockers should be used cautiously in patients with severe outflow obstruction, elevated pulmonary artery wedge pressure, and/or low systemic blood pressure because their vasodilatory action can worsen LVOT obstruction. The use of calcium channel blockers in combination with beta-blockers confers an increased risk of high-degree atrioventricular block and bradycardia, consequently preventing dose titration of the beta-blocker. Caution should also be exercised in patients with high gradients, advanced HF, or sinus bradycardia.26-28 Owing to their potent vasodilator effects, DHP calcium channel blockers should be avoided in symptomatic HCM patients with resting or provocable LVOT obstruction, severe systemic hypotension, or severe dyspnea at rest.50-57
Disopyramide: Disopyramide, a class Ia antiarrhythmic, is recommended in patients with obstructive HCM who remain symptomatic despite the use of beta-blockers and calcium channel blockers, alone or in combination. However, all three medications are rarely used concomitantly unless a permanent pacemaker is implanted to protect against complete heart block. Symptomatic benefit is thought to be due to disopyramide’s negative inotropic action.26-28
In small nonrandomized trials, disopyramide was found to improve clinical symptoms, LVOT gradients, and occurrence of sudden death.52-61 The optimal starting dosage for disopyramide controlled-release tablets is 250 mg given every 12 hours, and the dosage may be titrated to 600 mg daily in patients with a resting gradient 40 mmHg. Lower dosages may be used in patients with renal failure, with creatinine levels of 1.3 to 2.0, or weight <45.5 kg.62
Disopyramide should be initiated in the hospital with cardiac monitoring for potential arrhythmias or QT prolongation. The drug should be discontinued if the QTc interval is >525 ms. Its anticholinergic adverse effects can limit dose titration. Disopyramide used alone should be avoided in HCM patients with AF because it may enhance atrioventricular conduction and increase the ventricular rate during AF episodes.26,28,62
Amiodarone: Amiodarone, a class III antiarrhythmic, is widely used for AF although there are no prospective studies supporting its use in HCM. Amiodarone (150-400 mg daily) has demonstrated minimal effectiveness in preventing SCD, and its adverse-effect profile limits its use. Amiodarone is often used to control asymptomatic ventricular ectopic beats to prevent ICD discharge.26,28,63-66
Phenylephrine: IV phenylephrine, an alpha1 agonist, is recommended for the treatment of acute hypotension in obstructive HCM not responsive to fluid administration. In two case series, phenylephrine (0.5 mcg/kg/min titrated to response) was useful in patients who were acutely hypotensive owing to cardiogenic shock. Inotropes such as dopamine, dobutamine, and norepinephrine are potentially harmful and should not be used to treat acute hypotension in obstructive HCM.67-79
Diuretics: Oral diuretics can be used in both nonobstructive and obstructive symptomatic HCM when conventional therapy is ineffective at controlling symptoms of HF (i.e., dyspnea, congestion). Diuretics should be used judiciously since many HCM patients have diastolic dysfunction and require relatively high filling pressure to achieve adequate ventricular filling. Conventional treatment with loop diuretics is recommended, and the medication should be titrated to symptom relief.14,26,28
Treatment of AF in HCM
Patients with paroxysmal, persistent, or chronic AF in HCM should be managed with a vitamin K antagonist to a target international normalized ratio of 2 to 3 or with a direct thrombin inhibitor. Newer anticoagulant therapies have not been validated in this population; therefore, they are not recommended. Ventricular-rate control in patients presenting with AF with a rapid ventricular rate should be managed with beta-blocker and non-DHP calcium channel blocker therapy. Disopyramide with a rate-controlling agent or amiodarone is a reasonable antiarrhythmic for AF in HCM. Other antiarrhythmics, including sotalol, dofetilide, and dronedarone, may be considered, but a lack of evidence limits their utility.70-73
Investigational Studies
Many of the studies on the pharmacologic treatment of HCM were poorly conducted and were performed decades ago. There is a paucity of newer and more robust research despite recent discoveries about the disease process, increased awareness, and new technology, which can help identify larger patient populations. Several molecular targets have been identified and are currently being studied in animal models and in small trials in humans. The renin-angiotensin-aldosterone system (RAAS), specifically aldosterone and angiotensin II, has been identified as a target to reduce myocardial fibrosis and remodeling and improve myocardial ischemia. Several animal models and retrospective studies have shown preliminary positive results for RAAS inhibition.74-77 HCM is also associated with enhanced late sodium current causing intracellular calcium overload, which leads to abnormal energy handling and increased arrhythmogenicity. Ranolazine is a promising therapeutic option owing to its selective late sodium current inhibition, and studies are currently under way to determine the drug’s role in HCM.78,79
Conclusion
Hypertrophic cardiomyopathy is a cardiac contractile-muscle disorder most often caused by genetic mutations. Patients should be educated about genetic screening procedures and lifestyle-modification recommendations to prevent symptomatic disease occurrence. Comorbid conditions and risk factors should be aggressively managed to reduce the risk of symptomatic HCM. Symptomatic HCM treatment with beta-blockers and non-DHP calcium channel blockers is the mainstay of therapy. Appropriate treatment monitoring and medication education should be part of a global treatment approach. Patients should also be counseled to avoid medications that can exacerbate the degree of obstruction and thereby worsen symptoms. Pharmacists play an important role in educating patients on the importance of screening and adherence to lifestyle and treatment recommendations.
REFERENCES
1. Maron BJ, Gardin JM, Flack JM, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92:785-789.
2. Wynner JL, Braunwald E. Cardiomyopathy and myocarditis. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison’s Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill Medical; 2005:1408.
3. Maron MS. Overview of hypertrophic cardiomyopathy management including treatment of special problems. UpToDate. www.uptodate.com/contents/overview-of-hypertrophic-cardiomyopathy-management-including-treatment-of-special-problems?source=search_result&search=overview+of+hypertrophic+cardiomyoapthy&selectedTitle=2~150. Accessed January 15, 2016.
4. Ommen SR. Hypertrophic cardiomyopathy. Curr Probl Cardiol. 2011;36:409-453.
5. Taylor MR, Carniel E, Mestroni L. Familial hypertrophic cardiomyopathy: clinical features, molecular genetics and molecular genetic testing. Expert Rev Mol Diagn. 2004;4:99-113.
6. Marian AJ. Genetic determinants of cardiac hypertrophy. Curr Opin Cardiol. 2008;23:199-205.
7. Alcalai R, Seidman JG, Seidman CE. Genetic basis of hypertrophic cardiomyopathy: from bench to the clinics. J Cardiovasc Electrophysiol. 2008;19:104-110.
8. Ahmad F, Seidman JG, Seidman CE. The genetic basis for cardiac remodeling. Annu Rev Genomics Hum Genet. 2005;6:185-216.
9. Bos JM, Towbin JA, Ackerman MJ. Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:201-211.
10. Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104:557-567.
11. Frey N, Luedde M, Katus HA. Mechanisms of disease: hypertrophic cardiomyopathy. Nat Rev Cardiol. 2011;9:91-100.
12. Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308-1320.
13. Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28:1-83.
14. Wigle ED, Rakowski H, Kimball BP, Williams WG. Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation. 1995;92:1680-1692.
15. Aboulhosn J, Child JS. Left ventricular outflow obstruction: subaortic stenosis, bicuspid aortic valve, supravalvar aortic stenosis, and coarctation of the aorta. Circulation. 2006;114:2412-2422.
16. Maron MS, Olivotto I, Zenovich AG, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232-2239.
17. Panza JA, Petrone RK, Fananapazir L, Maron BJ. Utility of continuous wave Doppler echocardiography in the noninvasive assessment of left ventricular outflow tract pressure gradient in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1992;19:91-99.
18. Sasson Z, Yock PG, Hatle LK, et al. Doppler echocardiographic determination of the pressure gradient in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1988;11:752-756.
19. Cannon RO III, Rosing DR, Maron BJ, et al. Myocardial ischemia in patients with hypertrophic cardiomyopathy: contribution of inadequate vasodilator reserve and elevated left ventricular filling pressures. Circulation. 1985;71:234-243.
20. Maron MS, Olivotto I, Maron BJ, et al. The case for myocardial ischemia in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:866-875.
21. Maron BJ, Wolfson JK, Epstein SE, Roberts WC. Intramural (“small vessel”) coronary artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1986;8:545-557.
22. Frenneaux MP, Counihan PJ, Caforio AL, et al. Abnormal blood pressure response during exercise in hypertrophic cardiomyopathy. Circulation. 1990;82:1995-2002.
23. Fifer MA, Vlahakes GJ. Management of symptoms in hypertrophic cardiomyopathy. Circulation. 2008;117:429-439.
24. Wigle ED, Adelman AG, Auger P, Marquis Y. Mitral regurgitation in muscular subaortic stenosis. Am J Cardiol. 1969;24:698-706.
25. Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807-1816.
26. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e212-e260.
27. Spirito P, Seidman CE, McKenna WJ, Maron BJ. The management of hypertrophic cardiomyopathy. N Engl J Med. 1997;336:775-785.
28. Marian AJ. Contemporary treatment of hypertrophic cardiomyopathy. Tex Heart Inst J. 2009;36:194-204.
29. Maron BJ, Seidman CE, Ackerman MJ, et al. How should hypertrophic cardiomyopathy be classified?: What’s in a name? Dilemmas in nomenclature characterizing hypertrophic cardiomyopathy and left ventricular hypertrophy. Circ Cardiovasc Genet. 2009;2:81-85.
30. Cohen LS, Braunwald E. Amelioration of angina pectoris in idiopathic hypertrophic subaortic stenosis with beta-adrenergic blockade. Circulation. 1967;35:847-851.
31. Flamm MD, Harrison DC, Hancock EW. Muscular subaortic stenosis. Prevention of outflow obstruction with propranolol. Circulation. 1968;38:846-858.
32. Adelman AG, Shah PM, Gramiak R, Wigle ED. Long-term propranolol therapy in muscular subaortic stenosis. Br Heart J. 1970;32:804-811.
33. Thompson DS, Naqvi N, Juul SM, et al. Effects of propranolol on myocardial oxygen consumption, substrate extraction, and haemodynamics in hypertrophic obstructive cardiomyopathy. Br Heart J. 1980;44:488-498.
34. Ostman-Smith I, Wettrell G, Riesenfeld T. A cohort study of childhood hypertrophic cardiomyopathy: improved survival following high-dose beta-adrenoceptor antagonist treatment. J Am Coll Cardiol. 1999;34:1813-1822.
35. Gilligan DM, Chan WL, Joshi J, et al. A double-blind, placebo-controlled crossover trial of nadolol and verapamil in mild and moderately symptomatic hypertrophic cardiomyopathy. J Am Coll Cardiol. 1993;21:1672-1679.
36. Tendera M, Wycisk A, Schneeweiss A, et al. Effect of sotalol on arrhythmias and exercise tolerance in patients with hypertrophic cardiomyopathy. Cardiology. 1993;82:335-342.
37. Cabrera-Bueno F, García-Pinilla JM, Gómez-Doblas JJ, et al. Beta-blocker therapy for dynamic left ventricular outflow tract obstruction induced by exercise. Int J Cardiol. 2007;117:222-226.
38. Sherrid MV, Pearle G, Gunsburg DZ. Mechanism of benefit of negative inotropes in obstructive hypertrophic cardiomyopathy. Circulation. 1998;97:41-47.
39. Gistri R, Cecchi F, Choudhury L, et al. Effect of verapamil on absolute myocardial blood flow in hypertrophic cardiomyopathy. Am J Cardiol. 1994;74:363-368.
40. Udelson JE, Bonow RO, O’Gara PT, et al. Verapamil prevents silent myocardial perfusion abnormalities during exercise in asymptomatic patients with hypertrophic cardiomyopathy. Circulation. 1989;79:1052-1060.
41. Bonow RO, Frederick TM, Bacharach SL, et al. Atrial systole and left ventricular filling in hypertrophic cardiomyopathy: effect of verapamil. Am J Cardiol. 1983;51:1386-1391.
42. Bonow RO, Rosing DR, Bacharach SL, et al. Effects of verapamil on left ventricular systolic function and diastolic filling in patients with hypertrophic cardiomyopathy. Circulation. 1981;64:787-796.
43. Rosing DR, Condit JR, Maron BJ, et al. Verapamil therapy: a new approach to the pharmacologic treatment of hypertrophic cardiomyopathy: III. Effects of long-term administration. Am J Cardiol. 1981;48:545-553.
44. Rosing DR, Kent KM, Maron BJ, et al. Verapamil therapy: a new approach to pharmacologic treatment of hypertrophic cardiomyopathy. Chest. 1980;78(suppl 1):239-247.
45. Kaltenbach M, Hopf R, Kober G, et al. Treatment of hypertrophic obstructive cardiomyopathy with verapamil. Br Heart J. 1979;42:35-42.
46. McTaggart DR. Diltiazem reverses tissue Doppler velocity abnormalities in pre-clinical hypertrophic cardiomyopathy. Heart Lung Circ. 2004;13:39-40.
47. Sugihara H, Taniguchi Y, Ito K, et al. Effects of diltiazem on myocardial perfusion abnormalities during exercise in patients with hypertrophic cardiomyopathy. Ann Nucl Med. 1998;12:349-354.
48. Betocchi S, Piscione F, Losi MA, et al. Effects of diltiazem on left ventricular systolic and diastolic function in hypertrophic cardiomyopathy. Am J Cardiol. 1996;78:451-457.
49. Suwa M, Hirota Y, Kawamura K. Improvement in left ventricular diastolic function during intravenous and oral diltiazem therapy in patients with hypertrophic cardiomyopathy: an echocardiographic study. Am J Cardiol. 1984;54:1047-1053.
50. Senn M, Hess OM, Krayenbühl HP. [Nifedipine in the treatment of hypertrophic non-obstructive cardiomyopathy]. Schweiz Med Wochenschr. 1982;112:1312-1317.
51. Yamakado T, Okano H, Higashiyama S, et al. Effects of nifedipine on left ventricular diastolic function in patients with asymptomatic or minimally symptomatic hypertrophic cardiomyopathy. Circulation. 1990;81:593-601.
52. Hopf R, Thomas J, Klepzig H, Kaltenbach M. [Treatment of hypertrophic cardiomyopathy with nifedipine and propranolol in combination]. Z Kardiol. 1987;76:469-478.
53. Betocchi S, Cannon RO III, Watson RM, et al. Effects of sublingual nifedipine on hemodynamics and systolic and diastolic function in patients with hypertrophic cardiomyopathy. Circulation. 1985;72:1001-1007.
54. Yamakado T, Higashiyama S, Nakano T, Takezawa H. [Left ventricular systolic and diastolic functions in hypertrophic cardiomyopathy: with special reference to clinical symptom and the effects of calcium blocking agent (nifedipine)]. J Cardiol Suppl. 1987;16:53-63.
55. Cserhalmi L, Assmann I, Glavanov M, et al. [Long-term therapy of hypertrophic obstructive and non-obstructive cardiomyopathy with nifedipine in comparison to propranolol]. Z Gesamte Inn Med. 1984;39:330-335.
56. Paulus WJ, Lorell BH, Craig WE, et al. Comparison of the effects of nitroprusside and nifedipine on diastolic properties in patients with hypertrophic cardiomyopathy: altered left ventricular loading or improved muscle inactivation? J Am Coll Cardiol. 1983;2:879-886.
57. Lorell BH, Paulus WJ, Grossman W, et al. Modification of abnormal left ventricular diastolic properties by nifedipine in patients with hypertrophic cardiomyopathy. Circulation. 1982;65:499-507.
58. Pollick C, Kimball B, Henderson M, Wigle ED. Disopyramide in hypertrophic cardiomyopathy. I. Hemodynamic assessment after intravenous administration. Am J Cardiol. 1988;62:1248-1251.
59. Pollick C. Muscular subaortic stenosis: hemodynamic and clinical improvement after disopyramide. N Engl J Med. 1982;307:997-999.
60. Matsubara H, Nakatani S, Nagata S, et al. Salutary effect of disopyramide on left ventricular diastolic function in hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 1995;26:768-775.
61. Sherrid MV, Barac I, McKenna WJ, et al. Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;45:1251-1258.
62. Sherrid MV, Arabadjian M. A primer of disopyramide treatment of obstructive hypertrophic cardiomyopathy. Prog Cardiovasc Dis. 2012;54:483-492.
63. McKenna WJ, Oakley CM, Krikler DM, Goodwin JF. Improved survival with amiodarone in patients with hypertrophic cardiomyopathy and ventricular tachycardia. Br Heart J. 1985;53:412-416.
64. McKenna WJ, Harris L, Perez G, et al. Arrhythmia in hypertrophic cardiomyopathy. II: comparison of amiodarone and verapamil in treatment. Br Heart J. 1981;46:173-178.
65. Paulus WJ, Nellens P, Heyndrickx GR, Andries E. Effects of long-term treatment with amiodarone on exercise hemodynamics and left ventricular relaxation in patients with hypertrophic cardiomyopathy. Circulation. 1986;74:544-554.
66. Cecchi F, Olivotto I, Montereggi A, et al. Prognostic value of non-sustained ventricular tachycardia and the potential role of amiodarone treatment in hypertrophic cardiomyopathy: assessment in an unselected non-referral based patient population. Heart. 1998;79:331-336.
67. Haley JH, Sinak LJ, Tajik AJ, et al. Dynamic left ventricular outflow tract obstruction in acute coronary syndromes: an important cause of new systolic murmur and cardiogenic shock. Mayo Clin Proc. 1999;74:901-906.
68. Wigle ED, David PR, Labroose CJ, McMeekan J. Muscular subaortic stenosis; the interrelation of wall pressure, outflow tract “distending pressure” and orifice radius. Am J Cardiol. 1965;15:761-772.
69. Braunwald E, Ebert PA. Hemogynamic alterations in idiopathic hypertrophic subaortic stenosis induced by sympathomimetic drugs. Am J Cardiol. 1962;10:489-495.
70. Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101-e198.
71. Maron BJ, Olivotto I, Bellone P, et al. Clinical profile of stroke in 900 patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;39:301-307.
72. Connolly SJ, Ezekowitz MD, Yusuf S, et al.; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
73. Tendera M, Wycisk A, Schneeweiss A, et al. Effect of sotalol on arrhythmias and exercise tolerance in patients with hypertrophic cardiomyopathy. Cardiology. 1993;82:335-342.
74. Lim DS, Lutucuta S, Bachireddy P, et al. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation. 2001;103:789-791.
75. de Resende MM, Kriegel AJ, Greene AS. Combined effects of low-dose spironolactone and captopril therapy in a rat model of genetic hypertrophic cardiomyopathy. J Cardiovasc Pharmacol. 2006;48:265-273.
76. Tsybouleva N, Zhang L, Chen S, et al. Aldosterone, through novel signaling proteins, is a fundamental molecular bridge between the genetic defect and the cardiac phenotype of hypertrophic cardiomyopathy. Circulation. 2004;109:1284-1291.
77. Orenes-Piñero E, Hernández-Romero D, Jover E, et al. Impact of polymorphisms in the renin-angiotensin-aldosterone system on hypertrophic cardiomyopathy. J Renin Angiotensin Aldosterone Syst. 2011;12:521-530.
78. Olivotto I, Girolami F, Sciagrà R, et al. Microvascular function is selectively impaired in patients with hypertrophic cardiomyopathy and sarcomere myofilament gene mutations. J Am Coll Cardiol. 2011;58:839-848.
79. Wilson SR, Scirica BM, Braunwald E, et al. Efficacy of ranolazine in patients with chronic angina observations from the randomized, double-blind, placebo-controlled MERLIN-TIMI (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Segment Elevation Acute Coronary Syndromes) 36 trial. J Am Coll Cardiol. 2009;53:1510-1516.
To comment on this article, contact rdavidson@uspharmacist.com.





