US Pharm. 2014;39(2):51-54.
ABSTRACT: Metabolic syndrome comprises many risk factors, including—but not limited to—high blood sugar, hypertension, and dyslipidemia, the incidences of which are continuing to rise in the United States. It is important for pharmacists to be familiar with the various components of metabolic syndrome so that they can perform appropriate screenings in at-risk patients and encourage therapeutic lifestyle changes. Pharmacists can also make drug-therapy recommendations to help prevent further complications associated with metabolic syndrome.
Metabolic syndrome is a collection of co-occurring disorders that increase the risk of heart disease, stroke, and diabetes mellitus (DM). Metabolic risk factors such as elevated plasma glucose, high blood pressure (BP), and dyslipidemia contribute to atherosclerosis, as well as to a prothrombotic and proinflammatory state.1,2 Additional risk factors include abdominal obesity, physical inactivity, insulin resistance, cigarette smoking, and family history of premature coronary heart disease (CHD).2 Compared with patients who do not have metabolic syndrome, those with metabolic syndrome are twice as likely to develop CHD and five times more likely to develop DM.1-3 While the pathophysiology of metabolic syndrome is multifactorial, one component is the proinflammatory state, as denoted by an elevated level of C-reactive protein. In obesity, cytokine release from the adipose tissue causes inflammation, which leads to the development of dyslipidemia and increasing waist circumference.2,4
In the United States, metabolic syndrome affects approximately 35% of adults, of whom 85% also have DM.2,3 Prevalence, which varies by sex with respect to race and ethnicity, increases with age. Compared with non-Hispanic white males, non-Hispanic black males have a lower incidence of metabolic syndrome (37% vs. 25%). Non-Hispanic black and Mexican-American females are nearly 1.5 times more likely than non-Hispanic white females to have metabolic syndrome.5 Forty-one percent of males and 37% of females aged 40 to 59 years and 52% of males and 54% of females older than 60 years meet diagnostic criteria for metabolic syndrome.5
Diagnosis and Treatment
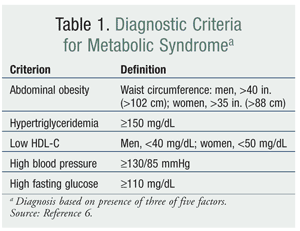
The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) outlines diagnostic criteria for metabolic syndrome (TABLE 1).6 Treatment for metabolic syndrome focuses on modification of these criteria. The primary treatment approach is nonpharmacologic, including weight reduction, dietary changes, physical activity, and smoking cessation.6 In addition to therapeutic lifestyle changes (TLC), patients may consider adjunct treatment with a weight-loss agent.
Weight-Loss Management
Medications such as Xenical or Alli (orlistat) or one of two recently approved weight-loss agents, Belviq (lorcaserin) and Qsymia (phentermine-topiramate extended-release), may be used adjunctively to manage weight loss. TABLE 2 presents these agents in greater detail.
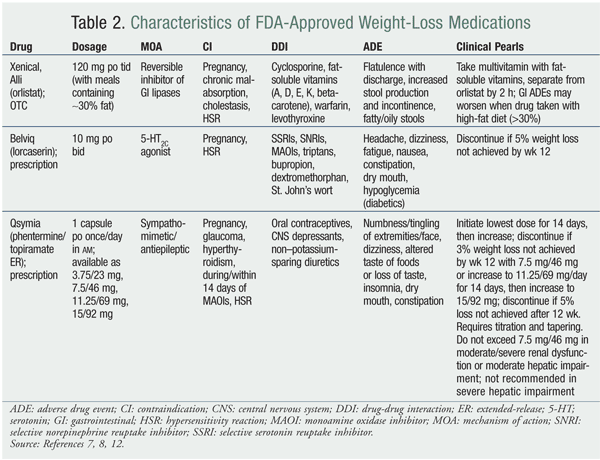
Orlistat: Xenical and Alli are indicated for weight loss and maintenance in obese patients and to reduce the risk of weight gain following a substantial weight loss. Orlistat exerts its effect on weight as a reversible inhibitor of gastrointestinal (GI) lipase enzymes. Since this drug acts locally within the GI tract, common side effects are GI in nature.7
Lorcaserin: Belviq is indicated as adjunct treatment in obese or overweight patients with comorbid conditions including hypertension, dyslipidemia, and type 2 DM (DM2). The CDC defines obesity as a BMI of ≥30.0 kg/m2 and overweight as a BMI of 25.0 to 29.9 kg/m2. Lorcaserin, an agonist of the serotonin 2C receptor, promotes satiety and decreases food consumption. Dosing is 10 mg twice daily. Side effects are minimal; however, patients with DM are more susceptible to hypoglycemia. If ≥5% weight loss is not achieved after 12 weeks, therapy should be discontinued. Lorcaserin is a Class IV controlled substance because of its potential for abuse. Patients should be advised to take this drug only as prescribed and never to share it.8
Three phase III clinical trials (BLOOM, BLOOM-DM, and BLOSSOM) investigated the safety and efficacy of lorcaserin in nondiabetic and diabetic subjects.9-11 The primary outcome was the percentage of subjects achieving ≥5% weight loss from baseline to week 52. In BLOOM, lorcaserin 10 mg twice daily was compared with placebo twice daily. The primary outcome was achieved in 47.5% of the lorcaserin group and 20.3% of the placebo group, respectively (P <.0001).9 BLOOM-DM compared both of these treatment modalities, plus lorcaserin 10 mg once daily. The once-daily group also received a placebo dose nightly for blinding purposes. The primary outcome was achieved in 37.5%, 44.7%, and 16.1% of the lorcaserin twice-daily, lorcaserin once-daily, and placebo groups, respectively (P <.001).10 BLOSSOM evaluated the same treatment modalities as BLOOM-DM. Higher percentages of subjects in the lorcaserin twice-daily (47.2%) and once-daily (40.2%) groups met the primary outcome, versus 25% of the placebo group (P <.0001).11
Phentermine/Topiramate Extended-Release: Although the exact mechanism of action of Qsymia is unknown, its components—a sympathomimetic and an extended-release antiepileptic—are thought to augment chronic weight management by increasing satiety and decreasing appetite. The indications are the same as those for lorcaserin. Dose titration is required upon initiation and discontinuation to prevent seizure occurrence. Dosing is 3.75/23 mg daily for 14 days, then 7.5/46 mg daily as maintenance. If 3% weight loss is not achieved after 12 weeks, therapy may be discontinued, or the dosage may be deescalated or increased to 11.25/69 mg daily for 14 days before being increased to a maximum of 15/92 mg daily. If 5% weight loss is not achieved after 12 weeks on the maximum dosage, therapy should be gradually discontinued. This product is available only through pharmacies enrolled in the Qsymia Certified Pharmacy Network.12
The safety and efficacy of phentermine/topiramate extended-release were evaluated in three phase III clinical trials (EQUIP, CONQUER, and SEQUEL).13-15 Over 52 weeks, EQUIP assessed severely obese subjects (BMI ≥35 kg/m2) and CONQUER studied overweight and obese subjects (BMI ≥27 to ≤45 kg/m2) with two or more comorbidities, such as hypertension, hyperlipidemia, DM, or increased waist circumference (women, ≥88 cm; men, ≥102 cm). The endpoint was ≥5% weight loss by trial completion. In EQUIP, subjects taking 3.75/23 mg and those taking 15/92 mg achieved an overall weight loss of 5.1% and 10.9%, respectively, versus 1.6% for the placebo group (P <.0001). In CONQUER, subjects taking 7.5/46 mg and those taking 15/92 mg achieved an overall loss of 7.8% and 9.8%, respectively, versus an overall loss of 1.2% in the placebo group (P <.0001).13,14 SEQUEL, a 52-week CONQUER extension study, evaluated long-term results of drug therapy, focusing on weight-loss maintenance during year 2. From baseline, 9.3% and 10.5% of subjects taking 7.5/36 mg and 15/92 mg, respectively, experienced weight-loss maintenance (P <.001).15
Management of Other Risk Factors
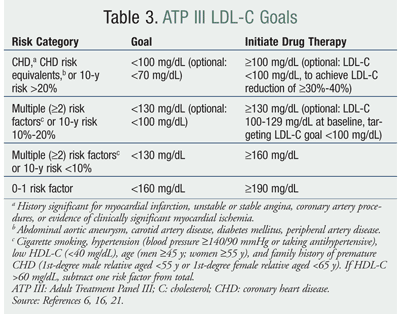
In addition to weight loss and/or maintenance, the other components of metabolic syndrome must be managed. In treating dyslipidemia, the primary goal is to lower LDL cholesterol (LDL-C). The target LDL-C depends upon the presence of CHD, risk equivalents, or risk factors. The ATP III LDL-C goal—as well as optional goals described in the 2004 update to the NCEP’s clinical practice guidelines—for each risk category appears in TABLE 3.6,16 To achieve goal LDL-C, TLC—a multifaceted lifestyle approach—must be implemented. In addition, drug therapy may be required to achieve the desired LDL-C. An HMG-CoA (statin) is recommended, not only for its LDL-C reduction potential, but also for its primary prevention of cardiovascular disease in high-risk patients and secondary prevention in patients with CHD.6
According to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), a number of TLC are warranted for BP reduction. These include the Dietary Approaches to Stop Hypertension (DASH) eating plan (emphasizing consumption of fruits, vegetables, fat-free or low-fat milk/dairy products, and whole grains); sodium restriction (≤2.4 g/day); weight reduction (to maintain BMI of 18.5-24.9 kg/m2); physical activity (regular aerobic activity for 30 minutes/day most days of week); moderate alcohol consumption (men, ≤2 drinks/day; women, ≤1 drink/day); and smoking cessation.17
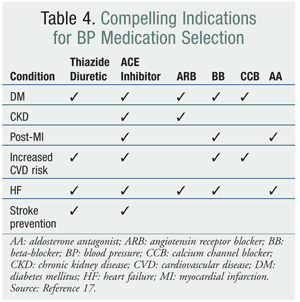
The JNC 7’s target BP goal for patients with chronic kidney disease or DM is <130/80 mmHg; for all others, it is <140/90 mmHg.17 The American Heart Association provides hypertension guidelines and goals for other categories. Target BP for patients with coronary artery disease (CAD) or CAD risk equivalents (carotid artery disease, peripheral artery disease, abdominal aortic aneurysm), as well as those at high risk for CAD (DM, chronic renal disease, or 10-year Framingham risk score ≥10%), is <130/80 mmHg. For ventricular dysfunction, the target BP goal is <120/80 mmHg.18 According to the JNC 7, drug-therapy selection is based upon compelling indications (TABLE 4).17
The 2013 American Diabetes Association guidelines recommend lifestyle modifications (similar to those in JNC 7) to reduce blood glucose (BG) and prevent DM2. Metformin also may be considered to treat elevated BG. Without a diagnosis of DM, the fasting BG goal is <100 mg/dL.19
Finally, since a proinflammatory state exists in metabolic syndrome, the use of aspirin should be considered. Aspirin 81 mg daily should be considered in patients with a 10-year risk ≥10%. Aspirin 81 to 325 mg daily should be used for secondary prevention in patients with a history of CHD.1
Pharmacist Interventions
Since most components of metabolic syndrome do not have associated symptoms, patients may not be concerned about risk. Pharmacists can host educational and screening programs to foster awareness of metabolic syndrome and identify at-risk patients who are candidates for therapy. Upon identification, patients should schedule a primary care appointment for further assessment and pharmacologic therapy.
Pharmacists can intervene further by setting measurable and achievable goals; assessing adherence; identifying barriers to treatment; emphasizing the role of nonpharmacologic therapy; supporting patients engaged in lifestyle modifications; and educating patients about safe medication use, risk-factor modification, diagnosis of metabolic syndrome, device use (glucometer or BP cuff), and lifestyle interventions. Pharmacists are in an ideal position to assess patients’ self-monitoring of weight, BG, and BP and help them develop care plans. As another component of the medication-therapy process, pharmacists can help patients develop and update a personal medication record.20 No matter which aspect is addressed, it is essential to engage patients during all encounters.
NOTE: Subsequent to the writing of this paper, new guidelines were issued by the Eighth Joint National Committee (2014), American Diabetes Association (2014), American College of Cardiology/American Heart Association (ACC/AHA) Task Force on Practice Guidelines [cholesterol treatment] (2013), and ACC/AHA/The Obesity Society [overweight/obesity management] (2013).
REFERENCES
1. Grundy SM, Cleeman JI, Daniels SR, et al; American Heart
Association; National Heart, Lung, and Blood Institute. Diagnosis and
management of the metabolic syndrome: an American Heart
Association/National Heart, Lung, and Blood Institute Scientific
Statement. Circulation. 2005;112:2735-2752.
2. Grundy SM, Brewer HB Jr, Cleeman JI, et al; American Heart
Association; National Heart, Lung, and Blood Institute. Definition of
metabolic syndrome: report of the National Heart, Lung, and Blood
Institute/American Heart Association conference on scientific issues
related to definition. Circulation. 2004;109:433-438.
3. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77-82.
4. Reilly MP, Rader DJ. The metabolic syndrome: more than the sum of its parts? Circulation. 2003;108:1546-1551.
5. CDC. NHANES III. 2009. www.cdc.gov/nchs/nhanes/nh3data.htm. Accessed August 30, 2013.
6. National Heart, Lung, and Blood Institute. Third Report of the
National Cholesterol Education Program (NCEP) Expert Panel on Detection,
Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult
Treatment Panel III). Final Report. NIH Publication No. 02-5215. September 2002. www.nhlbi.nih.gov/guidelines/cholesterol/atp3full.pdf. Accessed September 5, 2013.
7. Xenical (orlistat) product information. South San Francisco, CA: Genentech USA, Inc; December 2013.
8. Belviq (lorcaserin) product information. Woodcliff Lake, NJ: Eisai Inc; August 2012.
9. Smith SR, Weissman NJ, Anderson CM, et al; Behavioral Modification
and Lorcaserin for Overweight and Obesity Management (BLOOM) Study
Group. Multicenter, placebo-controlled trial of lorcaserin for weight
management. N Engl J Med. 2010;363:245-256.
10. O’Neil PM, Smith SR, Weissman NJ, et al. Randomized
placebo-controlled clinical trial of lorcaserin for weight loss in type 2
diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20:1426-1436.
11. Fidler MC, Sanchez M, Raether B, et al; BLOSSOM Clinical Trial
Group. A one-year randomized trial of lorcaserin for weight loss in
obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067-3077.
12. Qsymia (phentermine and topiramate extended-release) product information. Mountain View, CA: Vivus, Inc; September 2013.
13. Allison DB, Gadde KM, Garvey WT, et al. Controlled-release
phentermine/topiramate in severely obese adults: a randomized controlled
trial (EQUIP). Obesity (Silver Spring). 2012;20:330-342.
14. Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose,
controlled-release, phentermine plus topiramate combination on weight
and associated comorbidities in overweight and obese adults (CONQUER): a
randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341-1352.
15. Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss
and metabolic benefits with controlled-release phentermine/topiramate
in obese and overweight adults (SEQUEL): a randomized,
placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297-308.
16. Grundy SM, Cleeman JI, Merz CN, et al; Coordinating Committee of
the National Cholesterol Education Program. Implications of recent
clinical trials for the National Cholesterol Education Program Adult
Treatment Panel III guidelines. Circulation. 2004;110:227-239.
17. Chobanian AV, Bakris GL, Black HR, et al; Joint National
Committee on Prevention, Detection, Evaluation, and Treatment of High
Blood Pressure; National Heart, Lung, and Blood Institute; National High
Blood Pressure Education Program Coordinating Committee. Seventh report
of the Joint National Committee on Prevention, Detection, Evaluation,
and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
18. Rosendorff C, Black HR, Cannon CP, et al; American Heart
Association (AHA) Council for High Blood Pressure Research; AHA Council
on Clinical Cardiology; AHA Council on Epidemiology and Prevention.
Treatment of hypertension in the prevention and management of ischemic
heart disease: a scientific statement from the American Heart
Association Council for High Blood Pressure Research and the Councils on
Clinical Cardiology and Epidemiology and Prevention. Circulation. 2007;115:2761-2788.
19. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
20. American Pharmacists Association and the National Association of Chain Drug Stores Foundation. Medication Therapy Management in Pharmacy Practice: Core Elements of an MTM Service Model. Version 2.0. www.pharmacist.com/MTM/CoreElements2. Accessed August 30, 2013.
21. Last AR, Ference JD, Falleroni J. Pharmacologic treatment of hyperlipidemia. Am Fam Physician. 2011;84:551-558.
To comment on this article, contact rdavidson@uspharmacist.com.






