US Pharm. 2020;45(6)33-36.
ABSTRACT: Biological products are rapidly expanding in the United States, and they are currently the fastest-growing class of therapeutic products. Biological products are being developed for rare, difficult-to-treat disease states and disease states with no treatment options, as well as creating new options for treatable conditions. The FDA has identified potential safety concerns for patients using biological products. These concerns include inappropriate or unintended biological-product substitution, along with pitfalls in pharmacovigilance monitoring for biological products. Recent FDA guidance discusses concerns, proposes solutions, and describes nomenclature for naming biological products.
Biological medications are generally large, complex molecules. These characteristics create potential safety concerns, especially with the possibility of an immune response. These concerns led the FDA to create a guidance document for the nonproprietary naming of biological products.1 The document discusses the need for biological products to possess a nonproprietary name that contains a unique suffix. The intention of the FDA for adding the unique suffix to the proper name of each new biological product is to 1) prevent a patient from receiving a medication that was not the intended biological product prescribed and avoid alternation or switching of biological products not deemed interchangeable; 2) allow for manufacturer-specific pharmacovigilance tracking; and 3) implement these unique suffixes in ordering, prescribing, dispensing, and recordkeeping.1
This guidance was then open for public comment, and an updated version was subsequently released in March 2019.2 This article will discuss the FDA’s concerns and their proposed solutions, as well as the nomenclature used for biological product naming.
The World Health Organization (WHO) has developed multiple guidance documents for the naming of biological products. The first was released in 2011; the most recent editions from 2016 and 2017 will be referenced in this article. These documents focus on the naming of biological products, specifically regarding the biologic’s generic or proper name. The WHO documents describe how the proper name should be constructed to allow for uniformity between biological products.3,4
What Is a Biologic?
Biological products, also referred to as biologics, are created with biotechnology or another cutting-edge technology. Biological products encompass blood, blood components, somatic cells, gene therapy, tissues, recombinant proteins, and vaccines, and they are typically derived from microorganisms, plant, animal, or human cells.5,6 Biological products are composed of sugars, proteins, nucleic acids, or complex combinations of these substances.5,6
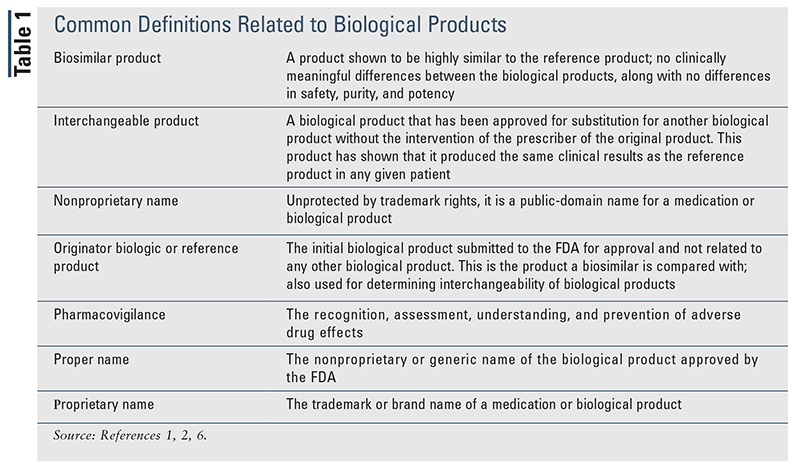
Biological products are different than most medications on the market. Most medications are small molecules, chemically synthesized, and their chemical structure is known. However, most biological products are complex, unique, large mixtures that are not readily identifiable or able to be exactly duplicated. Most biological products are also very sensitive to heat and vulnerable to microbial adulteration, thus requiring aseptic techniques from the initial manufacturing stages, another characteristic differentiating them from most conventional medications.5,6 These products may offer means to treat medical conditions currently having no treatment options or create new options for treatable disease states. Commonly used terminology regarding biological products is defined in TABLE 1.1, 2,6
Naming Guidelines for Biologics
As the number of biological products has increased, naming has become increasingly complex. The WHO has long been responsible for international nonproprietary name (INN) assignment. These nonproprietary (generic or proper) names refer to the unique active-ingredient component of the drug which can relate information about the chemical name or, in the case of the larger biological molecules, relate information about the nature of the complex molecule.7 This is not the proprietary (brand) name that is trademarked or registered for private use. The proper name aids healthcare professionals and patients in identifying the product and allowing products to be distinguished from each other.1
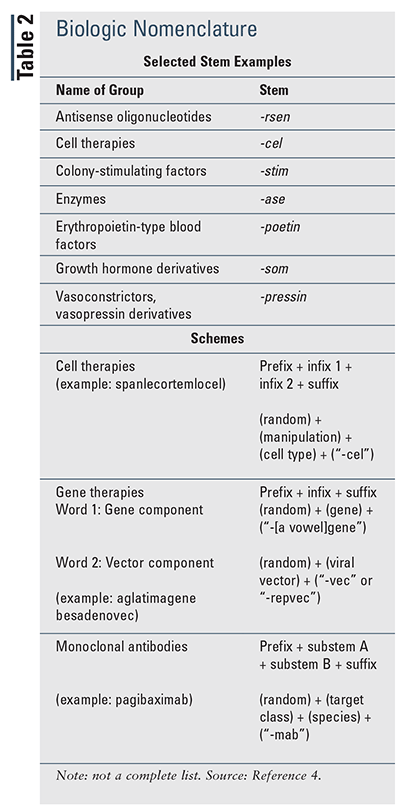
In 2017, the WHO updated guidance for using INNs, which included summary information on nomenclature for biologics. Specifically, naming of biologics is related to structure and/or function, and each is assigned a specific letter group or stem, although not all groups of biological agents have been assigned a stem (e.g., insulins).1 TABLE 2 includes examples of biological groups with their associated stem.4 There are also groups of biologics that have been assigned a scheme. Schemes include cell therapies, gene therapies, and monoclonal antibodies.4 The nomenclature schemes for these therapies with examples are included in TABLE 2.4
Biosimilars are a newer subset of biologics for which the system of assigning nonproprietary names has needed to be modified. While biosimilars are highly similar with no clinically meaningful differences, they are not automatically considered interchangeable. This is because unlike generic copies of nonbiological, small-molecule drugs, biosimilars are not exactly the same as the original or reference product. Since the process by which a biologic is produced is proprietary and the molecules are complex, biosimilars are expected to have minor differences when compared with the reference product.1
The FDA has published guidance on nonproprietary naming that addresses the issue of biosimilar and interchangeable products, and it has also published recommendations in 2019 on how it will assess interchangeability of biosimilar drugs.1,2 With the advent of biosimilars and potential for interchangeability, nonproprietary naming became even more complicated. The FDA recommends that moving forward, all biologics and their biosimilars share a core name with the reference product, which is the nonproprietary name under which the reference product was approved (e.g., filgrastim). In addition, each newly approved biologic and biosimilar should have a four-letter distinguishing suffix that is devoid of meaning. These four lowercase letters should be attached to the core name with a hyphen.1 For example, filgrastim-sndz, marketed under the brand name Zarxio, was the first FDA-approved biosimilar product. Multiple biosimilars that share the same core name each have a different distinguishing suffix. For example, adalimumab biosimilars are numerous and have the following distinguishing suffixes: -atto, -adbm, -adaz, -bwwd, and -afzb.8
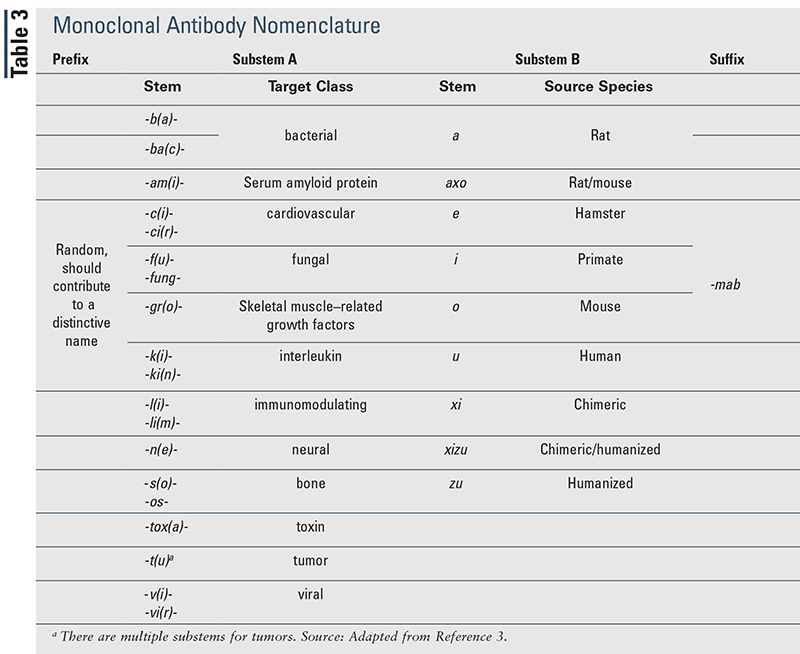
Monoclonal antibodies (mAbs) are a large subset of biologics that have their own specific naming scheme. The name consists of a prefix, substem A, substem B, and suffix (TABLE 3).3 The prefix is determined at random, chosen to contribute to pronounceability, and typically two to five letters in length. Substem A is based on the mAb target, such as a molecule, cell, or organ. The second letter (a vowel) in parenthesis is added only as needed to make the drug name easier to pronounce if substem B starts with a consonant. Substem B is based on the mAb source species from which the mAb is derived. A chimeric antibody has contiguous foreign-derived amino acids comprising the variable regions of both heavy and light chains linked to constant regions originating from a human. A humanized antibody is primarily derived from a human but has segments of foreign-derived amino acids interspersed.3 Using TABLE 3, a mAb that has a toxin target and is derived from a human might be called ‘bezlo-tox-u-mab,’ which is a marketed human mAb that binds toxins produced by Clostridium difficile.3
Scope of Biologic Naming
In the FDA’s 2017 guidance on biologic naming, the agency proposed retrospectively modifying the proper names of already licensed biological products by adding an FDA-designated suffix, but this is no longer the intention of the FDA.1,2 The agency is now proposing the addition of a unique suffix to biological products to new originator/reference, related, biosimilar, and interchangeable products.2 The FDA’s goal is to improve patient safety and allow for competition, lower prices, and greater access to biological products.2
Vaccines are discussed and fall under the scope of the FDA guidance on naming; however, the FDA is reconsidering this approach. The FDA is determining if the currently used system for vaccine administration is capable of ensuring safe dispensing and pharmacovigilance without creating distinguishable proper names for vaccines.2
Why Is This Important?
Prior to the implementation of these unique suffixes to the proper names of biological products, it was difficult to fully track adverse events for a specific manufacturer’s biologic if that product shared the same proper name of another biologic.1 The enhanced pharmacovigilance that is expected to result from these unique suffixes will allow for streamlined tracking of adverse events to a specific manufacturer, lot number, and/or manufacturing site.1 The unique suffix now allows for biological product differentiation.
There are concerns that many healthcare providers and patients assume that naming for biological products follows the same naming concepts as the naming of small-molecule drugs. If a small-molecule medication possesses the same generic name, they are more than likely interchangeable. However, this is not the case with biological products. A biological product possessing the same generic name does not infer interchangeability.2 The Purple Book includes guidance on all licensed biological products, including whether a biological product has been determined by the FDA to be interchangeable or if a biosimilar is interchangeable with the referenced product.9 The Purple Book was launched in 2014, is managed by the FDA, became searchable in February 2020, and is available at: purplebooksearch.fda.gov/.
Role of the Pharmacist
Pharmacists will play a vital role in the education of patients and healthcare providers regarding the new additions of unique suffixes to biological products. These unique suffixes will also allow pharmacists to assume a leadership role in the implementation and reporting of adverse-event information in reference to biological products. Pharmacists should also be familiar with the nomenclature of biological product name creation, as it will allow them to decipher new biological product origins and relative indication by breaking down the proper name. Pharmacists will also need to serve as experts on the Purple Book, a reference for all licensed biologics and approved biosimilar and interchangeable biological products.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. FDA. Nonproprietary naming of biological products—guidance for industry. 2017. www.fda.gov/downloads/Drugs/
GuidanceComplianceRegulatoryInformation/Guidances/UCM459987.pdf. Accessed March 24, 2020.
2. FDA. Nonproprietary naming of biological products—guidance for industry. Draft guidance. 2019. www.fda.gov/media/121316/download. Accessed March 24, 2020.
3. World Health Organization. International Nonproprietary Names (INN) for biological and biotechnological substances (a review). 2016. www.who.int/medicines/services/inn/BioReview2016.pdf. Accessed March 31, 2020.
4. World Health Organization. Guidance on the use of international nonproprietary names (INNs) for pharmaceutical substances. 2017. www.who.int/medicines/services/inn/innguidance/en/. Accessed March 31, 2020.
5. FDA. Center for Biologics Evaluation and Research (CBER). What are “biologics” questions and answers. 2018. www.fda.gov/about-fda/center-biologics-evaluation-and-research-cber/what-are-biologics-questions-and-answers. Accessed March 25, 2020.
6. FDA. Biological and interchangeable products. 2017. www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products. Accessed March 26, 2020.
7. Jones TD, Carter PJ, Plückthun A, et al. The INNs and outs of antibody nonproprietary names. mAbs. 2016;8(1):1-9.
8. FDA. Biosimilar product information. May 13, 2020. www.fda.gov/drugs/biosimilars/biosimilar-product-information. Accessed May 15, 2020.
9. FDA. Purple Book: Database of Licensed Biological Products. Updated May 11, 2020. https://purplebooksearch.fda.gov/. Accessed May 13, 2020.
To comment on this article, contact rdavidson@uspharmacist.com.