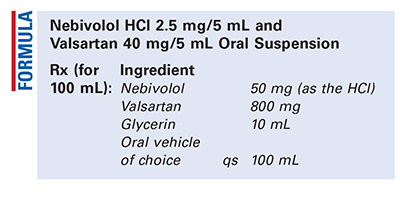
Method of Preparation: Calculate the quantity of each ingredient for the amount to be prepared. The commercial tablets contain 5 mg and 80 mg of nebivolol and valsartan, respectively, so 10 tablets may be used for this formula. Obtain the required number of tablets and thoroughly pulverize the tablets to a fine powder. Incorporate the glycerin and mix to form a smooth paste. Geometrically, add the oral vehicle of choice, with thorough mixing after each addition, to final volume. Package and label.
Use: This preparation has been used in the treatment of hypertension for patients who are unable to swallow the tablets.
Packaging: Package in tight containers.
Labeling: Keep out of reach of children. Use only as directed. Shake well. Store in a refrigerator. Discard after ____ [time period].
Stability: A USP nonsterile-preparations default beyond-use date of up to 14 days when stored in a refrigerator may be used for this preparation.1
Quality Control: Quality-control assessment can include volume, pH, specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, and physical stability (discoloration, foreign materials, gas formation, mold growth).2
Discussion: Byvalson (nebivolol and valsartan) is indicated for the treatment of hypertension to lower blood pressure. The oral liquid preparation given here is for patients who are unable to swallow oral solid tablets. Byvalson is available as a purple, capsule-shaped, film-coated tablet with “FL1” debossed on one side.3
Byvalson is available as tablets for oral administration, each containing nebivolol hydrochloride (HCl) 5.45 mg—equivalent to 5 mg of nebivolol free base—and valsartan 80 mg. The inactive ingredients in the tablets include lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, copovidone, colloidal silicon dioxide, magnesium stearate, talc, ferric oxide, hypromellose, polysorbate 80, and Opadry II Purple film-coat, which consists of polyvinyl alcohol (partially hydrolyzed), titanium dioxide, talc, polyethylene glycol, iron oxide red, and ferrosoferric oxide/iron oxide black.
Nebivolol HCl (C22H25F2NO4.HCl, MW 441.90) is present as a racemic mixture of d-nebivolol HCl and l-nebivolol HCl with the stereochemical designations of [SRRR]-nebivolol HCl and [RSSS]-nebivolol HCl, respectively. It occurs as a white to almost white powder that is soluble in methanol, dimethylsulfoxide, and N,N-dimethylformamide; sparingly soluble in ethanol, propylene glycol, and polyethylene glycol; and very slightly soluble in hexane, dichloromethane, and methylbenzene.3
Valsartan (C24H29N5O3, MW 435.52) occurs as a white or almost white, fine, hygroscopic powder. It is freely soluble in anhydrous ethanol, sparingly soluble in methylene chloride, and practically insoluble in water.3
Glycerin (glycerol, 1,2,3-propanetriol) occurs as a clear, colorless, odorless, viscous, hygroscopic liquid with a sweet taste about two-thirds as sweet as sucrose. It is used in ophthalmic formulations (0.5%-3% concentration) and as an antimicrobial preservative (>20% concentration), emollient, and humectant (up to 30% concentration); a plasticizer in film coating for tablets; a parenteral solvent (up to 50% concentration); and a sweetening agent in alcoholic elixirs (up to 20% concentration). Glycerin has a specific gravity of about 1.25 and a melting point of 17.8°C; if cooled to crystallization, it must be heated to about 20°C in order to melt. Glycerin is miscible with water, methanol, and 95% ethanol; practically insoluble in oils and chloroform; and slightly soluble in acetone. It is hygroscopic and should be stored in airtight containers in a cool place. Glycerin is not prone to oxidation, but it will decompose on heating. When mixed with water, ethanol, and propylene glycol, the mixtures are chemically stable. Incompatibilities include strong oxidizing agents that might cause the mixtures to explode, such as chromium trioxide, potassium chlorate, and potassium permanganate. When glycerin is mixed with phenols, salicylates, or tannin, a darkening of the mixtures may occur because of an iron contaminant in the glycerin.4
Regarding the oral vehicle of choice, a number of oral liquid vehicles are available that would be suitable for this formulation. Several vehicles are commercially available that are preserved and have a pH of 4 to 5, good viscosity, good pourability, good taste, and so on. The compounding pharmacist has the option of preparing the vehicle specifically according to the preferences of the patient.
REFERENCES
1. U.S. Pharmacopoeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; September 2017.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC. 1999;3:146-147.
3. RxList. Byvalson. www.rxlist.com/byvalson-drug.htm. Accessed September 11, 2017.
4. Alvarez-Nunez FA, Medina C. Glycerin. In: Rowe RC, Sheskey PJ, Cook WG, Fenton ME, eds. Handbook of Pharmaceutical Excipients. 7th ed. London, England: Pharmaceutical Press; 2012:324-327.
To comment on this article, contact rdavidson@uspharmacist.com.





