US Pharm. 2012;37(10):38-39.
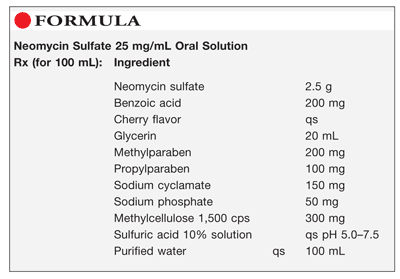
Method of Preparation: Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh or measure each ingredient. Heat about 75 mL of purified water to about 75°C; add the methylparaben, propylparaben, and methylcellulose 1,500, and mix until dissolved. Cool the solution to room temperature and add the neomycin sulfate, benzoic acid, glycerin, sodium cyclamate, sodium phosphate, and cherry flavor; mix until dissolved. Adjust the pH to the range of 5.0 to 7.5 using sulfuric acid 10% solution, if necessary. Add sufficient purified water to final volume and mix well. Package and label.
Use: Neomycin sulfate oral solution has been used for bowel preparation before abdominal surgery and for selective treatment of digestive-tract problems in patients in ICUs.
Packaging: Package in tight, light-resistant containers.1
Labeling: Keep out of the reach of children. Use only as directed. Shake well.
Stability: A beyond-use date of 14 days when stored in a refrigerator may be used for this preparation.1
Quality Control: Quality-control assessment can include weight/volume, pH, specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, and physical stability (discoloration, foreign materials, gas formation, mold growth).2
Discussion: Neomycin sulfate is an aminoglycoside antibiotic obtained from cultures of Streptomyces fradiae. It occurs as a white to slightly yellow hygroscopic powder that is freely soluble in water and very slightly soluble in alcohol. Neomycin sulfate has a potency equivalent of not less than 600 mcg of neomycin per mg, calculated on the dried basis.1
Benzoic acid (C7H6O2, MW 122.12) occurs as white crystals, scales, or needles. It has a slight odor, usually suggesting benzaldehyde or benzoin. Benzoic acid is freely volatile in steam, freely soluble in alcohol, and slightly soluble in water.1,3
Glycerin (glycerol, 1,2,3-propanetriol, C3H8O3, MW 92.10) occurs as a clear, colorless, odorless, viscous, hygroscopic liquid. It is miscible with water, methanol, and 95% ethanol. Glycerin is practically insoluble in oils and slightly soluble in acetone.4
Sodium cyclamate (C6H12NNaO3S, MW 201.22) occurs as white, odorless, or almost odorless crystals or as a crystalline powder with an intensely sweet taste. Sodium cyclamate is soluble 1 g in 5 mL of water, 1 in 25 of propylene glycol, and 1 in 250 of 95% ethanol. Its solutions are stable to heat, light, and air over a wide pH range.5
Sodium phosphate (Na3PO4, MW 163.94 [anhydrous], 181.96 [monohydrate], 380.13 [dodecahydrate]) occurs as white, odorless crystals or granules or as a crystalline powder. Sodium phosphate is freely soluble in water and insoluble in alcohol. The pH of a 1 in 100 solution is between 11.5 and 12.0.1,6
Methylcellulose (Methocel) is a practically odorless and tasteless, white to yellowish-white granule or powder. It is hygroscopic and is practically insoluble in acetone, ethanol, saturated salt solutions, and hot water. In cold water, methylcellulose swells and disperses to form a viscous, colloidal dispersion.7
Sulfuric acid (H2SO4, MW 98.08) contains, by weight, not less than 95.0% and not more than 98.0% of H2SO4. It is miscible with water and with alcohol with the generation of a lot of heat.8
REFERENCES
1. U.S. Pharmacopeia 35/National Formulary 30. Rockville, MD: US Pharmacopeial Convention, Inc; 2012:344-386,1103,1131,1145,1957-1958.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC. 1999;3:146-147.
3. Quinn ME. Benzethonium chloride. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:61-63.
4. Alvarez-Nunez FA, Medina C. Glycerin. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:283-286.
5. Goggin PL. Sodium cyclamate. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:643-645.
6. Kearney AS. Sodium phosphate, dibasic. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:656-658.
7. Allen LV Jr, Luner PE. Methylcellulose. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:438-441.
8. Amidon GE. Sulfuric acid. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. London, England: Pharmaceutical Press; 2009:719-720.
To comment on this article, contact rdavidson@uspharmacist.com.





