US Pharm. 2023;48(1):8-12.
ABSTRACT: Lyme disease is a common vector-borne illness most often caused by Borrelia burgdorferi in infected ticks. Ticks are small and difficult to see. They may go unnoticed for extended periods of time, allowing for the transmission of Lyme disease to its host. A hallmark of Lyme disease is the presentation with erythema migrans, or the “bull’s-eye” rash at the site of a tick bite. If left untreated, Lyme disease can progress to impact the heart (Lyme carditis), joints (Lyme arthritis), and the peripheral and central nervous systems (neurologic Lyme disease). In patients who present with neurologic Lyme disease, first-line treatment options include IV ceftriaxone, cefotaxime, penicillin G, or oral doxycycline. Pharmacists can recommend appropriate drug therapy for patients and provide patient education to prevent Lyme disease.
According to the CDC, Lyme disease is the most common vector-borne disease in the United States.1 Lyme disease is typically caused by Borrelia burgdorferi and rarely by Borrelia mayonii.1 Although there is no way to track exactly how many people get Lyme disease each year, state and local health departments report cases of Lyme disease to the CDC through the Nationally Notifiable Diseases Surveillance System (NNDSS).2 From this information, the CDC estimates approximately 300,000 people are infected each year, despite only 35,000 cases being reported to NNDSS, which is a passive system that relies on healthcare providers to submit records.2 The top 10 states that reported Lyme disease in 2020 include Connecticut, Maine, Maryland, Massachusetts, Minnesota, New Hampshire, New Jersey, New York, Pennsylvania, and Wisconsin. The mid-Atlantic states have consistently reported the highest number of cases for the past 10 years.3
Transmission
B burgdorferi is spread via the bite of infected ticks. Blacklegged ticks, also known as deer ticks (Ixodes scapularis), spread Lyme disease in the northeastern, mid-Atlantic, and north-central U.S. In the Pacific Coast, the western blacklegged tick (Ixodes pacificus) spreads disease. Ticks attach to the body in areas such as the groin, armpits, and scalp. They are hard to see, as most humans are infected by immature ticks, known as nymphs, in the spring and summer. Nymphs are less than 2 mm, about the size of a poppy seed. Adult ticks also have the ability to spread disease, but they are larger, about the size of a sesame seed, and are therefore more easily seen and removed before they can transmit bacteria. A tick must be attached for 36 to 48 hours or longer before the disease can be transmitted. Lyme disease does not spread from person-to-person, nor can dogs and cats spread disease directly to their owners. Ticks cannot fly or jump. Blacklegged ticks live for about 2 years. There are four life stages: egg, larva, nymph, and adult. At each life-cycle stage, the tick must have a blood meal to survive. Ticks can feed from a variety of hosts, including mammals, birds, and reptiles, and they need a new host at each life-cycle stage.
Once attached to a host, depending on the species and stage of life, preparing to feed takes 10 minutes to 2 hours. Its saliva contains anesthetic properties, so its host cannot feel that the tick has attached itself. Ticks will suck blood slowly for several days from a host and transmit Lyme disease. If a tick is removed within 24 hours, there is a significant reduction in the likelihood of Lyme disease transmission.4 Ticks can be removed with a fine-tipped tweezer by grasping the tick as close as possible to the skin. The tick should be pulled upward steadily and should not be twisted or jerked, as parts can break off and remain in the skin. It is important to try to remove the tick mouth parts with tweezers. After removal, the bite area should be cleaned with rubbing alcohol or soap and water. The live tick can be disposed of by putting it in alcohol, putting it in a sealed bag or container, wrapping it in tape, or flushing it down the toilet. The tick can also be sent for testing for Lyme disease.5
Clinical Presentation
Early signs and symptoms of Lyme disease will occur 3 to 30 days after the tick bite. Patients may experience fever, chills, headache, fatigue, muscle and joint aches, and swollen lymph nodes. The most prominent sign is the erythema migrans rash or “bull’s-eye” rash, occurring in 70% to 80% of infected people. It begins at the bite, appearing in about 7 days, and expands over time to as large as 12 or more inches in diameter. It is rarely itchy or painful and does not always appear as a classic erythema migrans rash. It can be challenging to identify a tick bite since a small bump or redness at the site occurs immediately, resembling a mosquito bite. Ticks can also spread other diseases, causing different types of rashes. Later signs and symptoms can occur in days to months.6
The following may occur as complications of Lyme disease if left untreated: Neurologic Lyme disease can impact the peripheral or central nervous systems; cranial nerve involvement results in facial palsy on one or both sides of the face; peripheral nerve involvement results in numbness, tingling, or shooting pain and weakness in the extremities; and central nervous system involvement with Lyme meningitis causes fever, headache, and neck stiffness. In 100 reported CDC cases, nine had facial palsy, four had radiculopathy, and three had meningitis or encephalitis. Two-step serologic testing is recommended for neurologic Lyme disease. Cerebral spinal fluid is not necessary for diagnosis but can exclude other sources of infection.7 Lyme arthritis is characterized by severe joint pain and swelling in the knee and other large joints. Lyme arthritis accounts for one out of four reported Lyme disease cases. If left untreated, permanent joint damage can occur.8 Lyme carditis is marked by heart palpitations or irregular heartbeat, dizziness, and shortness of breath. Lyme carditis occurs in about one of 100 Lyme disease cases reported to the CDC.9
Lyme disease can be treated in about 2 to 4 weeks (further discussed in Treatment section); however, some patients continue to experience symptoms that include pain, fatigue, or difficulty thinking that can endure for more than 6 months. This is known as post-treatment Lyme disease syndrome (PTLDS), and the cause is unknown despite treatment. It is proposed that an autoimmune response is triggered by B burgdorferi similar to responses that occur after chlamydia or strep throat. Others propose there is continued infection that is undetected or symptoms are associated with another disease unrelated to Lyme disease. There is no treatment for PTLDS, and prolonged antibiotic courses have not proven to be beneficial.10
Diagnosis
The CDC recommends a two-step process for testing via a blood sample. If the first step is negative, no additional testing is needed. If the first step is positive or indeterminate, the second step should be completed. The overall results for Lyme disease are considered positive if the first test is positive and the second test is positive. Notable aspects about Lyme testing are that it detects antibodies and, since it can take several weeks to develop antibodies, a recently infected patient can test negative. Infections with other diseases can cause false positives.11
Treatment
In 2020, the Infectious Diseases Society of America, American Academy of Neurology, and American College of Rheumatology published guidelines for the prevention, diagnosis, and treatment of Lyme disease.12 Within these guidelines, recommendations for the management of neurologic Lyme disease can be found. In patients with acute neurologic manifestations of Lyme disease, such as those presenting with Lyme-associated meningitis, cranial neuropathy, radiculoneuropathy, or other peripheral nervous system manifestations, IV ceftriaxone, cefotaxime, penicillin G, or oral doxycycline are recommended as preferred therapy (TABLE 1). The guidelines recommend selecting among the preferred antimicrobials based on factors such as route of administration, side effect profile, ability to tolerate oral medication, and compliance likelihood, as efficacy among the different regimens has been demonstrated to be similar.13,14 In patients with parenchymal involvement of the brain or spinal cord, such as encephalitis or myelitis, IV therapy should be used over oral therapy. The recommended treatment duration for patients with neurologic Lyme disease is 14 to 21 days, and the treatment route of administration may be changed from IV to oral during therapy.12
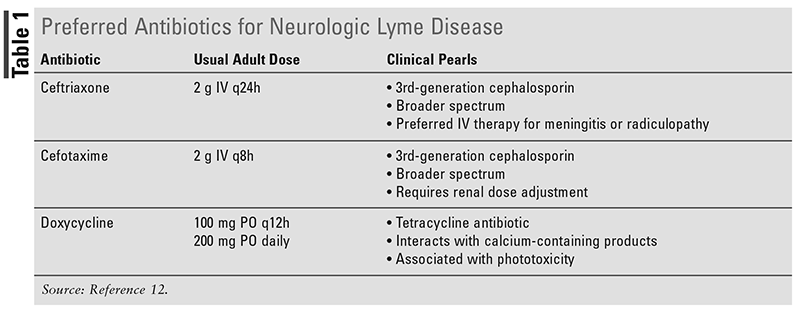
Of the preferred antibiotic agents for treatment, ceftriaxone and cefotaxime are broader spectrum. Both IV ceftriaxone and cefotaxime are third-generation cephalosporin antibiotics that inhibit bacterial cell wall synthesis by binding to penicillin-binding proteins. They have microbiologic activity against common gram-positive bacteria such as methicillin-susceptible Staphylococcus aureus and Streptococcus pneumoniae and against gram-negative bacteria, such as Enterobacterales.15,16 Cefotaxime also has activity against anaerobic bacteria, such as Bacteroides spp.16 The recommended dosage of ceftriaxone in neurologic Lyme disease is 2 grams IV every 24 hours. The 2-gram dosing is recommended over 1-gram dosing to achieve higher concentrations within the central nervous system. Common adverse effects associated with ceftriaxone administration include dermatologic reactions, nausea, vomiting, and diarrhea.15 The recommended dosage of cefotaxime in neurologic Lyme disease is 2 grams IV every 8 hours in persons with normal renal function. Dosage adjustments are needed for those with altered renal function. Common adverse reactions associated with cefotaxime use are also dermatologic and gastrointestinal in nature.16
IV penicillin G is the narrowest spectrum of the recommended antibiotic agents against neurologic Lyme disease and works by interfering with bacterial cell wall synthesis.17 Its spectrum of activity is primarily gram-positive but also has activity against syphilis and other spirochetal infections, such as Lyme disease. High-dose IV penicillin G is needed for treatment, with the recommended dosage of 18 million to 24 million units per day in divided doses given every 4 hours. Dosage adjustments for renal impairment are necessary. Adverse reactions may include dermatologic effects, gastrointestinal effects, and hypersensitivity.17
Doxycycline is a tetracycline antibiotic that inhibits protein synthesis by binding to the 30S and possibly 50S ribosomal subunits of bacteria. It has microbiologic activity against gram-positive bacteria, including methicillin-resistant S aureus and some gram-negatives. The recommended dosage of doxycycline for neurologic Lyme disease is 100 mg by mouth every 12 hours or 200 mg by mouth every 24 hours. Doxycycline absorption may be decreased by iron and calcium, and therefore it should be taken on an empty stomach 1 hour before or 2 hours after meals. Adverse reactions associated with doxycycline use are esophageal injury, photosensitivity, and gastrointestinal effects.18 Traditionally, doxycycline has been avoided in children younger than age 8 years and in pregnant or breast-feeding women due to concerns of staining of permanent teeth; however, these data are primarily based on older tetracyclines. More recent data support doxycycline as being safe when used short term in young children.19
Role of the Pharmacist
Pharmacists can play many roles in management of patients with neurologic Lyme disease. As medication experts, pharmacists are uniquely positioned to help recommend the most appropriate of the preferred agents based on patient-specific factors. For example, in a patient hospitalized with neurologic Lyme disease, high-dose IV ceftriaxone may be preferred because of its once-daily dosing. However, in a person with mild neurologic Lyme disease being managed in the community, doxycycline may be preferred because of its oral availability.
Along with helping select initial therapy, pharmacist expertise can be utilized to provide patient education and perform antibiotic adherence counseling. A recent study showed that intensive pharmacist counseling and call-back services led to significantly improved antibiotic adherence rates and high rates of symptom resolution.20 Pharmacists may also assist in identifying patients who can be transitioned from IV to oral therapy. Criteria for transitioning from IV to oral therapy are generally based on the patients’ overall clinical status, ability to tolerate oral medication, and presence of a functional gastrointestinal tract. Benefits of transitioning from IV to oral therapy include reduction in costs, reduction in hospital stays, and reduction in IV line complications.21 For example, a patient started on treatment for neurologic Lyme disease with ceftriaxone 2 grams IV daily may be transitioned to oral therapy when they are clinically stable (e.g., afebrile, leukocytosis improving, symptoms improving), able to tolerate oral therapy, and have a functional gastrointestinal tract to complete their 14-to-21-day course.
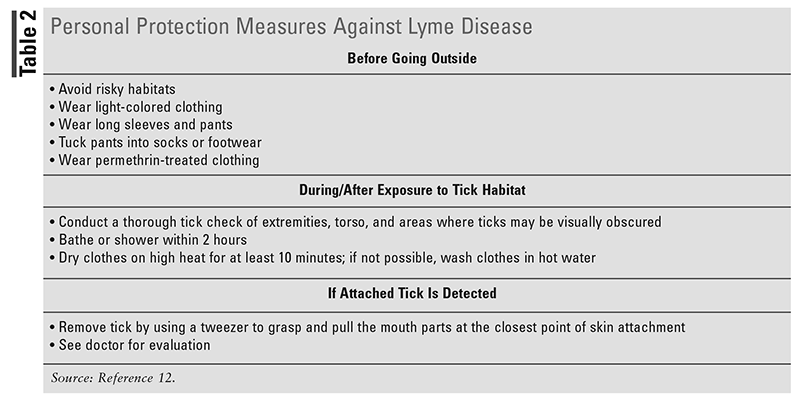
Another key role that pharmacists play in managing Lyme disease is identifying patients who are at risk for infection and recommending appropriate prevention methods.22 Ticks that carry Lyme disease typically live in moist, humid environments, near grassy or wooded areas. Therefore, individuals who spend time outdoors in regions with a high prevalence of ticks infected with Lyme may be at higher risk for infection.23 Pharmacists can recognize this and counsel patients who are at risk to perform daily tick checks and use personal protective measures and repellents to prevent tick bites (TABLE 2). Personal protective measures include avoiding risky habitats, wearing light-colored clothing, and wearing long sleeves and pants. Recommended repellents for the prevention of tick bites include N,N-diethylmeta-toluamide (DEET), picaridin, ethyl-3-(N-n-butyl-N-acetyl) aminopropionate (IR3535), oil of lemon eucalyptus (OLE), p-Menthane-3,8-diol (PMD), 2-undecanone, or permethrin.12 Each product should be used according to the manufacturer’s instructions.
Conclusion
Neurologic Lyme disease is a treatable disease that can be prevented with personal protective measures. Pharmacists in both the inpatient and outpatient settings can help provide services to assist patients. Inpatient pharmacists can make pharmacologic recommendations and adjustments while community pharmacists can provide medication counseling on the importance of medication adherence to prevent PTLDS and education for preventing Lyme disease.
REFERENCES
1. CDC. Lyme Disease. January 2022. www.cdc.gov/lyme/index.html.
2. CDC. How many people get Lyme disease. January 2021. www.cdc.gov/lyme/stats/humancases.html.
3. CDC. Surveillance Data. October 2022. www.cdc.gov/lyme/datasurveillance/surveillance-data.html.
4. CDC. Transmission. January 2020. www.cdc.gov/lyme/transmission/index.html.
5. CDC. Tick removal and testing. May 2022. www.cdc.gov/lyme/removal/index.html.
6. CDC. Signs and symptoms of untreated Lyme disease. January 2021. www.cdc.gov/lyme/signs_symptoms/index.html.
7. CDC. Neurologic Lyme disease. August 2021. www.cdc.gov/lyme/treatment/NeurologicLyme.html.
8. CDC. Lyme arthritis. October 2021. www.cdc.gov/lyme/treatment/LymeArthritis.html.
9. CDC. Lyme carditis. February 2022. www.cdc.gov/lyme/treatment/lymecarditis.html.
10. CDC. Post-treatment Lyme disease syndrome. January 2022. www.cdc.gov/lyme/postlds/index.html.
11. CDC. Diagnosis and testing. May 2021. www.cdc.gov/lyme/diagnosistesting/index.html.
12. Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the IDSA, AAN, and ACR: 2020 guidelines for the prevention, diagnosis, and treatment of Lyme Disease. Clin Infect Dis. 2021;72(1):e1-e48.
13. Bremell D, Dotevall L. Oral doxycycline for Lyme neuroborreliosis with symptoms of encephalitis, myelitis, vasculitis or intracranial hypertension. Eur J Neurol. 2014; 21:1162-1167.
14. Karlsson M, Hammers-Berggren S, Lindquist L, et al. Comparison of intravenous penicillin G and oral doxy for treatment of Lyme neuroborreliosis. Neurology. 1994;44(7):1203-1207.
15. Ceftriaxone. Package Insert. Basel, Switzerland: Sandoz. 2013. www.accessdata.fda.gov/drugsatfda_docs/label/2014/065169s022lbl.pdf. Accessed October 21, 2022.
16. Cefotaxime. Package Insert. Paris, France: Sanofi-Aventis, 2015. www.accessdata.fda.gov/drugsatfda_docs/label/2015/050547s071,050596s042lbl.pdf Accessed October 21, 2022.
17. Penicillin G. Package Insert. Deerfield, IL: Baxter Healthcare Corp. 2016. www.accessdata.fda.gov/drugsatfda_docs/label/2016/050638s019lbl.pdf. Accessed October 21, 2022.
18. Doxycycline. Package insert. Canonsburg, PA: Mylan. 2016. www.accessdata.fda.gov/drugsatfda_docs/label/2016/90431Orig1s010lbl.pdf. Accessed October 19, 2022.
19. Pöyhönen H, Nurmi M, Peltola V, et al. Dental staining after doxycycline use in children. J Antimicrob Chemother. 201.
20. Paravattil B, Zolezzi M, Nasr Z, et al. An interventional call-back service to improve appropriate use of antibiotics in community pharmacies. Antibiotics (Basel). 2021;10(8):986.
21. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016 May 15;62(10):e51-e77.
22. Jen C, Dorado V, Lu B, et al. Lyme disease: the role of the pharmacist in treatment and prevention. US Pharm. 2016;41(4):22-26.
23. CDC. Prevent Lyme disease. April 2022. www.cdc.gov/ncezid/dvbd/media/lymedisease.html#:~:text=People%20living%20in%20or%20visiting,your%20risk%20of%20Lyme%20disease.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





