US Pharm. 2006;10:10-14.
Research and Development (R & D) investments in new drugs in the United States by biotechnology and pharmaceutical companies reached a record $51.3 billion in 2005, up from $37 billion in 2004, according to the Pharmaceutical Manufacturers' Association. The increased investment in biomedical R & D in 2005 continues 25 years of strong growth--from $2 billion in 1980. The R & D investments far exceeded those of the international pharmaceutical industry and the NIH. The steady growth of R & D investments continues to support important advances in better drugs and new treatments for patients.
Drug Development and Approval
Process
The United States
has the most rigorous process in the world for new drug approvals. It takes 10
to 15 years for an experimental drug to become available to patients. It is
estimated that only five in 5,000 compounds that enter preclinical testing
make it to human testing. And only one of those five is approved for
marketing. It costs a company $802 million to get one new drug from the
laboratory to patients. Once a compound is identified to have a potential
beneficial to the patient, it undergoes preclinical testing (i.e., animal
testing) to determine how safe it is. After completion of the animal testing,
the company files an Investigational New Drug Application with the FDA,
seeking approval to start testing in humans.
Toxicity, dosage, and efficacy are determined through clinical trials, which have three phases. Phase I tests are conducted in 20 to 100 healthy human volunteers. The compound's absorption, distribution, metabolism, and excretion are determined. The next phase tests the compound in 100 to 500 volunteers with the condition the drug is intended to treat in order to determine its efficacy. In phase III trials, the compound is administered to 1,000 to 5,000 patients, who are carefully monitored by physicians who are testing to confirm efficacy and help identify adverse events. Following these three phases, favorable review of the data by the FDA permits the drug to be marketed to patients.
Neurological Disease Drugs
Studies show that
older patients are particularly susceptible to many neurological diseases,
including Alzheimer's disease, epilepsy, Parkinson's disease, and stroke.
These diseases cost the U.S. economy hundreds of billions of dollars each year
in treatment, lost wages, and reduced productivity. Drugs now in development
for neurological disorders include:
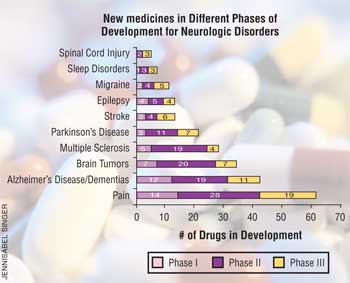
• 61 for pain--57% of U.S. adults experienced chronic or recurrent pain in the past year; Pain costs $100 billion each year.
• 34 for brain tumors--In 2004 alone, 40,900 new primary brain tumors (malignant and benign) were diagnosed. Brain tumors are the second leading cause of cancer-related deaths in children. Annually, 3,140 Americans younger than 20 are diagnosed with primary brain tumors, which are the most common form of solid tumor in children and the second most frequent malignancy of childhood.
• 42 for Alzheimer's disease--Found in 4.5 million Americans, including one in 10 people older than 65 and nearly half of those older than 85, this disease is may affect between 11.3 million and 16 million Americans by 2050. Each year, $100 billion is spent on Alzheimer's disease.
• 21 for Parkinson's disease--This disease affects 1.5 million Americans. Each year, 60,000 new cases are diagnosed.
• 13 for stroke--The third leading cause of death after heart disease and cancer, stroke affects about 700,000 Americans every year. In adults older than 55, the lifetime risk of stroke is greater than one in six. Stroke accounted for more than one of every 15 deaths. The direct and indirect costs of stroke were about $56.8 billion in 2005.
• 11 drugs for migraine headache--this condition affects 28 million people, or 13% of the U.S. population. Migraines affect both men and women, although they are three times more common in women than in men. An estimated 157 million workdays are lost each year because of the pain and associated symptoms of migraine, costing U.S. employers $13 billion a year in missed work and reduced productivity.
• 28 drugs for multiple sclerosis--This disease affects roughly 400,000 Americans, and every week another 200 are diagnosed. Up to three times as many women as men have multiple sclerosis. Most people are diagnosed between the ages of 20 and 50.
• 13 drugs for epi lepsy--This disease affects two million Americans. About 20% of seizures in children are due to cerebral palsy or other neurological abnormalities; and
• Seven drugs for sleep disorders--Insomnia affects more than 70 million Americans. Chronic or severe insomnia affects up to 15% of adults. Some 12 million Americans experience restless legs syndrome, which increases with age. Sleep deprivation and sleep disorders are estimated to cost Americans $100 billion annually in lost productivity, medical expenses, sick leave, and property and environmental damage.
Promising new drugs in the pipeline include a drug that uses normal human cells to enhance brain levels of dopamine, the neurotransmitter deficient in patients with Parkinson's disease, and a drug for brain cancer that singles out and latches onto and destroys the receptors on the surface of the malignant cells. As these new drugs undergo testing, patients wait eagerly for a cure, remission, prolongation of life span, and improved quality of life.
To comment on this article, contact editor@uspharmacist.com.






