US Pharm. 2011;36(6):58-62.
Over the past few months, the FDA has developed a different strategy for going after drug manufacturers that allegedly violate FDA mandates, such as prohibiting the marketing of approved drugs for off-label use. During the past several years, the FDA has accepted heavy settlement fees or court judgments, sometimes running into the hundreds of millions and even billions of dollars.
Fines Levied Against Pharmaceutical Companies
For example, Pfizer paid $1.3 billion in criminal fines for “misbranding” drugs, the legal term for off-label marketing—the largest amount levied so far.1 And that was just the tip of the iceberg, considering the total package of the settlement was $2.3 billion when adding in Department of Justice (DOJ) fees and costs. The fine was so high because Pfizer was considered a “repeat offender” after it continued to promote Lipitor and Viagra for off-label uses following a request to cease and desist these practices by the FDA.
In an earlier case from 2007, Purdue Pharma, together with three company executives, agreed to plead guilty to misleading the public in its marketing approach for the narcotic drug OxyContin. The company also agreed to pay fines and other assessments totaling $600 million.2 At the time, that was the largest criminal penalty ever leveraged against a company. The three executives who pled guilty were also excluded from doing business with the federal government for 15 years.3
In a case decided in 2009, a drug representative from Pharmaco pled guilty to telling approximately 100 employees she supervised in 2006 that they should promote the formerly available drug Bextra for pain and other purposes not approved by the FDA.4 She accepted personal responsibility for the misinformation, agreed to pay a $75,000 fine, and received 2 years’ probation.
In another case, GeneScience Pharmaceutical, a Chinese company, and its chief executive officer (CEO) pled guilty in October 2010 to federal charges of illegally distributing human growth hormone in the United States.5 This conviction concluded a 3-year investigation of the organization. In the U.S. District Court in Providence, Rhode Island, they agreed to pay $3 million toward a “Clean Competition Fund” that would support drug-free sports and $7.2 million in criminal forfeitures. The company founder, Lei Jin, entered a guilty plea and was sentenced to 5 years’ probation. The company pled guilty to a felony.
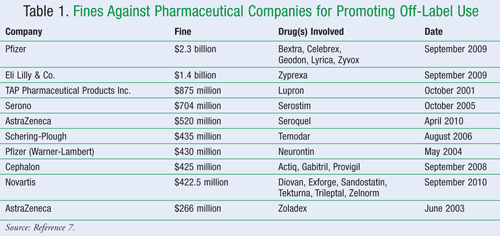
The only problem with reprimanding drug manufacturers with large monetary penalties is that many manufacturers seem to consider the fines as just another cost of doing business for misdeeds while collecting huge sums of profit from the allegedly illegal acts.6 A summary of such fines can be found in TABLE 1.7
When the judgment and settlement penalties proved to be ineffective deterrents of these practices, the FDA and DOJ started to find other ways to try to stop the manufacturers from ongoing unlawful activities. The federal agencies have begun to employ two new strategies: 1) bring criminal charges against wayward company executives, and 2) bar the offending manufacturers from doing any business with or for the various and multiple federal prescription benefit plans (e.g., Medicare, Medicaid). The second prong of this strategy would put nearly every manufacturer subject to this policy out of business because the federal government is the largest single purchaser of drugs in the world. The first prong, however, has its limitations, as evidenced by a recent judgment by a court hearing allegations of wrongdoing against a corporate official.
Facts of the Case
On May 10, 2010, a federal district court judge dismissed a complaint against a high-ranking attorney for GlaxoSmithKline (GSK).8 Most unusually, the judge threw out the case even before letting the jury deliberate, stating that this case should never have been brought in the first place and that it would be a miscarriage of justice were the defendant found guilty of the charges against her. This was the first time in over 7 years on the bench that this judge tossed out the complaint when the prosecution rested its case.
Prosecutors alleged that the attorney obstructed an FDA investigation into whether the company improperly promoted the antidepressant Wellbutrin SR for the off-label purpose of weight loss. The prosecution claimed that the in-house attorney for the company lied during an investigation, denying that she had any knowledge that the company was promoting the drug for an off-label use. The defense claimed she acted in a legitimate and zealous manner to protect the manufacturer and that she justifiably relied in good faith on the advice of an outside law firm.
In his opinion dismissing the case, the judge stated, “I conclude on the basis of the record before me that only with a jaundiced eye and with an inference of guilt that’s inconsistent with the presumption of innocence could a reasonable jury ever convict this defendant.”9
It is almost impossible to successfully prosecute an attorney representing a client for a criminal offense because the vast majority of communications between a client and the lawyer are privileged, meaning that no one can compel the disclosure of the content of information discussed by the parties. The judge noted that there is an “enormous potential for abuse in prosecuting an attorney giving advice to a client.” This loss for the DOJ is going to make it more difficult to prosecute individual corporate leaders for alleged misconduct. As a procedural matter, the government cannot appeal the case or seek reversal in an appellate court.
While there have been a few drug company executives who have pled guilty to criminal and/or civil charges relating to the unlawful marketing of a product, this strategy of suing corporate executives, who almost always rely on the advice of their attorneys, is very problematic. Overcoming an attorney-client privileged communication, especially in drug company cases, will be all the more difficult following this decision.
One of the most disturbing aspects of this case is that it appears the DOJ was attempting to force GSK into a monetary settlement for the alleged wrongful marketing practices by threatening to prosecute a corporate officer. This form of intimidation by any federal, state, or local prosecutor should be barred from even getting into a courthouse. In rendering his decision, the judge stated that privileged and confidential information between a client and his or her lawyer were the only basis for the charges against the defendant. He appeared to be incensed that a trial court magistrate had compelled discovery of those privileged documents and was unabashedly critical of the prosecutors who had introduced them into evidence.
Similar Cases
In one example where the DOJ was successful with this strategy, the former chairman of the board and CEO of St. Louis–based KV Pharmaceutical Company pled guilty and was sentenced in a case involving KV’s production and distribution of oversized morphine sulfate tablets.10 The U.S. district judge from the Eastern District of Missouri ordered the individual to pay a $1 million fine, forfeit $900,000, and serve a sentence of 30 days in jail. Note that the CEO pled guilty to only two misdemeanor violations of the Food, Drug, and Cosmetic Act (FDCA). In a plea agreement that was submitted to the court, the former CEO admitted that KV introduced misbranded morphine sulfate tablets into interstate commerce in 2007 and 2008. The government charged that the morphine sulfate included some oversized tablets, which contained more active ingredient of the drug morphine than was specified in its labeling. This made the morphine sulfate “misbranded” under federal law. Part of the plea agreement bars the CEO from doing business with the federal government for 20 years; he immediately resigned his post so that the company could avoid exclusion from government sales. The sentence handed down in that case would have been far more severe if the former CEO had pled guilty to felony charges.
The CEO was also an officer of the Ethex Corporation, a subsidiary of KV. In a related case, the DOJ filed criminal charges against Ethex in March 2010, which pled guilty to two felony offenses as a result of its failure to file required reports with the FDA concerning certain oversized drug tablets. The company was ordered to pay $28.1 million in fines, forfeitures, and restitution.11
This strategy of barring convicted drug companies and/or their executive officers from doing any business with the federal government has raised a firestorm of complaints and concerns about how pharmaceutical companies do business. In DOJ language, the barring of the company from doing business with the federal government is known as an “exclusion order” and is used when officers prove to be “untrustworthy individuals.”12 In the KV and Ethex cases, the government barred the companies from selling their products to any federal agency unless or until the convicted CEO resigned or was terminated from the company.
In another case that has Wall Street in a tizzy, the Department of Health and Human Services (HHS) has excluded Forest Laboratories from selling products to the federal government until its 83-year-old CEO of more than 34 years resigns. However, the company recently pled guilty to federal charges that its sales force illegally marketed the antidepressants Celexa and Lexapro to children and adolescents, even though these drugs had not been approved for minors. The company agreed to pay $313 million to settle criminal and civil charges.13
In a separate case, the DOJ has also been investigating GSK for misbranding and similar issues in the promotion of the diabetes drug Avandia over a fairly long period of time, but no charges have been filed as a result of this investigation. However, GSK has reserved (set aside) up to $6 billion to settle allegations that the drug could cause cardiac problems and strokes.14
Criticism of the Strategy
The DOJ has come under criticism from some commentators questioning “whether regulators have overreached, bypassing the court system in favor of banishing drug company executives without having to prove a case against them.” Lewis Morris, chief counsel of the HHS inspector general, testified before Congress in February 2011 that the agency’s exclusion authority was “one of the most powerful tools in our arsenal.”15
Perhaps these recent developments are not news to pharmacies and insurers or other providers of prescription drug benefits. The HHS has used its exclusionary authority in more than 30 cases since 1996, typically against employees of pharmacies and billing services, rather than executives of large organizations such as pharmaceutical companies and medical device manufacturers. “We are concerned that the providers that engage in health care fraud may consider civil penalties and criminal fines a cost of doing business,” Morris said. “By excluding the individuals who are responsible for the fraud, either directly or because of their positions or responsibility in the company that engaged in fraud, we can influence corporate behavior without putting patient access to care at risk.”16
This position also comes with some critiques. Jackson Nickerson, a professor of organization and strategy at Washington University’s Olin Business School, said the agency’s “underlying model” for exclusion appeared to be an example of overregulation. He went on to state, “It’s predicated on the assumption that if a company has done something wrong, it must mean the senior executives have done something wrong. You are branded as being unethical if a subordinate makes a decision that turns out to be a bad one.” He also stated that “to encourage innovation, company executives needed to delegate certain responsibilities to employees lower down in the organization. The dilemma is, how do you engage in global competition and decentralize, while at the same time be held accountable as an individual leader?”17
Nevertheless, proponents of using the exclusionary process and criminal penalties for company executives who either know about or actively participate in promoting drugs for off-label purposes are getting support from Congress. The U.S. Sentencing Commission proposed in January 2011 to use the Patient Protection and Affordable Care Act as a means to impose stricter sentencing guidelines, assuring that senior executives of drug companies acting outside of FDA constraints will see higher fines and more imprisonment time as well as lengthened exclusionary periods.18
Analysis
It has been uniformly accepted that physicians may prescribe and pharmacists may dispense drugs for uses that are not indicated on the FDA-approved labeling as long as there is a general consensus of opinion that the off-label use is scientifically viable, as indicated in studies usually published in peer-reviewed journals, and that the use is not experimental. The cases described here demonstrate that while an off-label use might well be accepted, the manufacturer cannot promote a drug for indications that have not been approved by the FDA. While there are only a few situations where a pharmacist might know that a prescribed drug is being used for an off-label purpose, because a diagnosis is rarely required on a prescription, pharmacists must take care not to become willing participants with overzealous drug companies that are promoting drugs without FDA approval.
REFERENCES
1. Pfizer to pay $1.3 billion criminal fine for misbranding its drugs. Natural News. www.rawpeople.com/index.php?
2. Pharmaceutical company pleads guilty to fraud involving marketing drug OxyContin. InjuryBoard BlogNetwork. May 21, 2007. http://phoenix.injuryboard.
3. Doyle J. Fed’s move to oust drug company chief. STL Today. April 22, 2011. www.stltoday.com/business/
4. Drug sales executive pleads guilty in Bextra case. Monforton and Partners. May 30, 2009. www.bextra-lawsuit.ca/news/
5. Wilson D. Drug maker from China pleads guilty. NY Times. October 8, 2010. www.nytimes.com/2010/10/07/
6. Are fines just an “expense” of doing business for Rx drugs ? World of DTC Marketing. April 26, 2011. http://worldofdtcmarketing.
7. Id. Used with permission from Richard Meyer, World of DTC Marketing. May 17, 2011.
8. Mundy A, Kendall B. U.S. rebuffed in Glaxo misconduct case. Wall Street Journal. May 11, 2011. http://online.wsj.com/article/
9. Hensley S. Federal judge acquits ex-Glaxo lawyer before defense even starts. National Public Radio. May 10, 2011. www.scpr.org/news/2011/05/10/
10. Former drug company executive pleads guilty in oversized drug tablets case. DOJ News. March 10, 2011. www.justice.gov/opa/pr/2011/
11. See Note 10, supra.
12. See Note 3, supra.
13. See Note 3, supra.
14. Glaxo’s (legal) trials and tribulations. Rocket Lawyer. May 11, 2011. http://legallyeasy.
15. See Note 3, supra.
16. See Note 3, supra.
17. See Note 3, supra.
18. Proposed law seeks stronger punishments for pharmaceutical executives. About Lawsuits. April 15, 2011. www.aboutlawsuits.com/
To comment on this article, contact rdavidson@uspharmacist.com.





