US Pharm. 2008;33(3)(OTC suppl):4-7.
Asthma is a pulmonary disease characterized
by reversible airflow obstruction and bronchial hyperresponsiveness. The
central feature of asthma is inflammation, which leads to recurrent episodes
of wheezing, coughing, shortness of breath, and chest tightness.1,2
The goal of therapy is to gain control of the disease in order to maintain a
normal quality of life.
The burden of asthma has been steadily
increasing over the past three decades. In 1970, approximately 3% of the
population had asthma, compared with 5.5% and 7.7% in 1995 and 2005,
respectively.3 The latter estimate equates to approximately 22.2
million Americans.2,3 With an increase in the prevalence of this
disease in the United States, morbidity and mortality due to asthma are also
increasing. Attributed to asthma in 2004 were 1.8 million emergency-department
visits, 497,000 hospitalizations, and 4,055 deaths.3
Treatment Based on Asthma Control
The National Asthma Education and
Prevention Program (NAEPP), a division of the National Heart, Lung, and Blood
Institute, publishes Guidelines for the Diagnosis and Management of Asthma
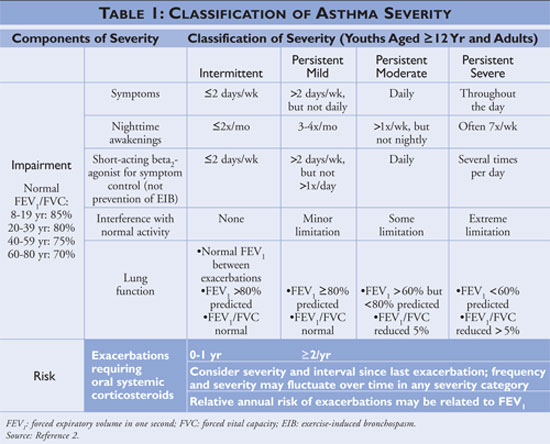
, most recently updated in 2007.2 The guidelines classify asthma
severity as intermittent or persistent based on symptoms and frequency of use
of quick-relief medications. Individuals with intermittent asthma have
infrequent symptoms, consequently infrequent use of quick-relief medication,
and no interference with normal activity. If the asthma is less controlled,
then the asthma severity is classified as persistent (see TABLE 1).
The guidelines describe the cornerstones of
asthma management as self-management education, the use of quick-relief
medications, and the use of long-term control medications in individuals with
persistent asthma. Health care providers offering self-management education
should instruct patients to identify and avoid asthma triggers when possible,
use pharmacologic therapies properly, and devise an asthma action plan.
Quick-relief medication should be initiated for all patients diagnosed with
asthma and may be used as monotherapy in individuals with intermittent asthma;
individuals diagnosed with persistent asthma, however, should have one or more
long-term control medications added to their regimen. Long-term control
medications include inhaled corticosteroids, leukotriene modifiers,
long-acting beta-agonists, and theophylline (see TABLE 2).

The guidelines also note that approximately
30% of the U.S. population uses complementary and alternative medications and
interventions. At present, however, insufficient evidence exists to recommend
these treatment modalities for asthma. The guidelines advise against the use
of herbal remedies to treat asthma based on the lack of safety and efficacy
data.
Nonprescription Asthma Products and Their
Regulation
Nonprescription asthma products have been
available since the 1950s, although some of the active ingredients have
changed considerably over the years. Currently available nonprescription
active ingredients are ephedrine--with or without guaifenesin--and epinephrine.
Theophylline and phenobarbital previously were active ingredients in
nonprescription asthma products; however, both are now classified as
prescription-only products.4
For the past three decades, the FDA has
taken actions regarding the regulation of nonprescription treatments for
asthma. In 1976, the FDA concluded that inhaled epinephrine provided quick and
effective relief in mild to moderate asthma. In 1982, it again concluded in a
tentative final monograph: "Epinephrine, epinephrine bitartrate, and
epinephrine hydrochloride (racemic; since renamed racepinephrine
hydrochloride) in pressurized metered-dose inhalation aerosol dosage forms can
be generally recognized as safe and effective for OTC use at dosage for adults
and children 4 years of age and older of 1 to 2 inhalations of a metered-dose
equivalent to 0.16 to 0.25 mg epinephrine per inhalation not more often than
every 3 hours."5,6
In 1986, the FDA reviewed ephedrine products
for bronchodilation and approved this active ingredient as generally
recognized as safe and effective for nonprescription use. In that year, the
FDA decided that data were insufficient to limit inhaled epinephrine to
prescription use only and that the benefits of nonprescription availability
outweighed the risks. The FDA concluded that expanded and revised labeling
would benefit consumers.5,6
After reports of life-threatening events and
deaths attributable to nonprescription combination products containing
theophylline, the FDA issued a final ruling in 1995 that these products were
not safe or effective.7 Thus, products containing a combination of
ephedrine and theophylline were discontinued and some were reformulated to
contain ephedrine and guaifenesin. Also in 1995, the FDA proposed to remove
ephedrine from the nonprescription market due to its role in the manufacture
of methamphetamine and methcathinone and its misuse for weight control and
muscle enhancement.7
In 1996, the FDA amended the 1986 final
monograph to remove monograph status for OTC bronchodilators.6
Instead, manufacturers of any new nonprescription metered-dose inhaler are
required to submit an approved application that contains information not found
in the monograph.6 Currently marketed nonprescription
bronchodilators were not affected by this amendment.
Despite the FDA's proposal to remove
ephedrine from the nonprescription market because of its use as a base element
in methamphetamine production, the product remains available nonprescription.
Instead, the Combat Methamphetamine Epidemic Act of 2005, incorporated into
the Patriot Act in 2006, conferred behind-the-counter status on
ephedrine-containing nonprescription products.8 Consumers wishing
to purchase products containing ephedrine now must present government-issued
photo identification at a pharmacy, as these products are no longer legally
sold in convenience stores or warehouse stores without a registered pharmacy.
As part of global public-health protection,
an international agreement was formed to reduce and eventually eliminate
products that contain ozone-depleting substances (ODSs) such as
chlorofluorocarbons (CFCs) found in metered-dose inhalers (MDIs). This
agreement gave rise to the 1987 Montreal Protocol and subsequently the 1990
Clean Air Act Amendments.9-11 The agreement provides an exemption
for the use of CFCs in MDIs used for asthma treatment if use of the product is
determined to be essential by the FDA.12 Nonprescription asthma
medications have remained under the essential-use designation. However, in
2007 the FDA announced a proposal to remove the essential-use designation for
inhaled epinephrine effective December 31, 2010.13 The FDA used the
following criteria, found in 21 CFR 2.125(g)(2), to review the essential-use
designation for inhaled epinephrine: "(i): Substantial technical barriers
exist to formulating the product without ODSs; (ii): The product will provide
an unavailable important public health benefit; and (iii): Use of the product
does not release cumulatively significant amounts of ODSs into the atmosphere
or the release is warranted in view of the unavailable important public health
benefit."13 The FDA is currently receiving comments on this
matter prior to their final ruling.
Safety and Efficacy of Nonprescription
Products
As evidenced by the various proposals made and
actions taken by the FDA regarding the nonprescription status of asthma
products, the past few decades have seen much debate regarding the safety and
efficacy of the active ingredients in these products. Although these products
are not actively promoted, their availability means that their safety and
efficacy are critically important for consumers.
Ephedrine: Single-ingredient
ephedrine products for use as bronchodilators have been considered generally
recognized as safe and effective by the FDA since 1986.14 Ephedrine
releases epinephrine from tissues, which triggers alpha-adrenergic and
nonselective beta-adrenergic receptor activity. The effectiveness of ephedrine
for bronchodilation has been demonstrated in studies dating to the 1950s.
Properties that make ephedrine less desirable as a bronchodilator include a
slower onset of action than inhaled products (15 minutes to one hour) and
nonselectivity of beta stimulation, which could contribute to cardiovascular
adverse events (AEs).15-17 However, studies have demonstrated
conflicting results regarding cardiovascular adverse events attributable to
ephedrine use at labeled doses of 12.5 to 25 mg.16
Epinephrine: Epinephrine
exhibits the same alpha- and beta-adrenergic receptor activity as ephedrine;
therefore the same concerns exist as to the possibility of cardiovascular AEs.
The onset of action of inhaled epinephrine (15 sec) is much more rapid than
that of oral ephedrine, making it a more appropriate choice for immediate
relief from an acute asthma exacerbation.6 Its short duration of
action (23 min), however, may necessitate that the patient use the product
repeatedly for continued relief rather than following the label instructions
of no more than two inhalations within three hours.6
Nonprescription epinephrine inhalers appear to be safe and effective for
patients with mild, intermittent asthma when used as labeled.5 This
remains a source of controversy, however, as many health care providers are
concerned that patients who rely on nonprescription inhalers may not be
receiving appropriate disease management from a health care team, possibly
leading to severe repercussions.
Guaifenesin: Guaifenesin is
used in combination with ephedrine for asthma treatment and is recognized as
safe and effective when the product is labeled for cough associated with
asthma.14 Although guaifenesin is an FDA-approved mucolytic, it
does not possess bronchodilatory effects. Thus, it contributes no additional
benefit to the ephedrine product for an acute exacerbation.17
Use of Nonprescription Asthma Products
A Wyeth Consumer Healthcare (WCH)
survey conducted in 1999 found that roughly six million (30%) individuals with
asthma use epinephrine inhalers.6 Respondents' self-reports suggest
that about 20% of these six million would be classified as having
mild-to-moderate persistent asthma, which is not in the labeled use for this
product.5 The NAEPP guidelines promote the use of short-acting beta
agonists at least as needed by all individuals suffering from asthma; the
guidelines do not specifically mention inhaled epinephrine as a treatment
choice, however.
In 2005, WCH conducted an Internet survey of
330 individuals regarding the use of nonprescription bronchodilators for
treatment of their symptoms. WCH determined that, of nonprescription-only
users, 92% had been diagnosed with asthma, 91% used inhaled epinephrine only
when experiencing an acute asthma exacerbation, 31% had no medical insurance,
and 38% had no prescription insurance.6 WCH extrapolated these
results to estimate that approximately 5% to 10% of individuals with asthma
use nonprescription bronchodilators as monotherapy.6 The primary
reasons given for nonprescription use--ease of access and lower cost--directly
correlate with health care providers' concerns that individuals using
nonprescription asthma products may not be receiving appropriate disease
management.6
Discussion
The properties desirable in acute
asthma treatment include effectiveness as a bronchodilator, safeness when
taken as labeled, fast onset of action, and long duration of action. Both
inhaled epinephrine and oral ephedrine have demonstrated efficacy in trials
for decades. Given the mixed cardiovascular results in clinical trials,
however, the safety of nonprescription bronchodilator use by individuals who
may not be followed medically is a major concern of health care providers.
Inhaled epinephrine has a faster onset of action than oral ephedrine, but its
short duration of action may limit its effectiveness.
The availability of oral ephedrine has been
limited since 2006 by its behind-the-counter status. The availability of
inhaled epinephrine hinges on the FDA's decision regarding whether this
formulation will maintain its essential-use designation. No nonprescription
alternatives exist for currently marketed CFC-containing nonprescription
bronchodilators.12 One manufacturer tried to reformulate its
nonprescription inhaled-epinephrine product with a hydrofluoroalkane
propellant, with undesirable effects for the user; it has partnered with
another pharmaceutical firm for reformulation in anticipation of filing with
the FDA by 2011.6 Key concerns regarding alternative propellants
include the need for effective delivery of epinephrine and safety and efficacy
data for the new product as a result of the changes.18
In determining whether to remove the
essential-use designation for inhaled epinephrine, the FDA is not examining
the safety or efficacy of the currently marketed CFC-containing products;
rather, it is examining the impact on public health should the designation be
removed. In letters from the National Association of Chain Drug Stores and the
American Pharmacists Association to the FDA on the proposal to remove the
essential-use designation, the primary concern was the potential negative
impact on current users of nonprescription inhalers if the products became
unavailable.19,20 The FDA has stated that if the proposed rule to
remove the essential-use designation becomes final and nonprescription
alternatives do not exist, it will be necessary for consumers to obtain a
prescription for an alternative product.12 Many consumers using
inhaled epinephrine may choose to forego asthma treatment or seek alternative
nonprescription therapy.
It is imperative that the medical community
make the public aware of the seriousness of the consequences of uncontrolled
asthma and that any person who experiences difficulty breathing should see a
health care provider for diagnosis and treatment. A patient who chooses to use
nonprescription bronchodilators should be advised to inform his or her health
care provider; the patient also should be counseled on the difference in label
instructions between nonprescription and prescription inhalers. The current
continued accessibility of nonprescription bronchodilators necessitates the
informed counseling of patients to prevent improper use of these products and
subsequent adverse effects.
REFERENCES
1. Moorman JE, Rudd RS, Johnson
CA, et al. National Surveillance for Asthma--United States, 1980–2004.
MMWR. Oct 19, 2007;56:1-14, 18-54.
2. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Asthma Education and Prevention Program. Bethesda, MD: National Heart, Lung, and Blood Institute, National Institutes of Health; 2007.
3. Akinbami L. Asthma prevalence, health care use and mortality: United States, 2003-05. National Center for Health Statistics. www.cdc.gov/nchs/products/pubs
/pubd/hestats/ashtma03-05/asthma03-05.htm. Accessed December 10, 2007.
4. W. Steven Pray. OTCproducts and asthma therapy. US Pharm. 1997;22(7):18-22.
5. Dickinson BD, Altman RD, Deitchman SD, Champion HC. Safety of over-the-counter inhalers for asthma: report of the Council on Scientific Affairs. Chest. 2000;118:522-526.
6. Wyeth ConsumerHealthcare. 2005N–0374. Use of ozone-depleting substance: essential-use determination of over-the-counter (OTC) epinephrine metered dose inhalers. Briefing document. December 19, 2005. www.fda.gov/ohrms/dockets/ac
/06/briefing/2006-4200B1_01_01-Wyeth-Backgrounder.pdf. Accessed December 30, 2007.
7. Kupec IF. Theophylline-containing bronchodilators and ephedrine drug products. Answers T95-40. July 27, 1995. www.fda.gov/bbs/topics/ANSWERS/ANS00675.html. Accessed December 10, 2007.
8. Drug Enforcement Administration. General information regarding the Combat Methamphetamine Epidemic Act of 2005 [Title VII of Public Law 109-177]. May 2006. www.deadiversion.usdoj.gov/meth/cma2005_general_info.pdf. Accessed February 4, 2008.
9. Jarabek AM, Fisher JW, Rubenstein R, et al. Mechanistic insights aid the search for CFC substitutes: risk assessment of HCFC-123 as an example. Risk Anal. 1994;14:231-250.
10. DeCanio SJ, Norman CS. Economics of "essential use exemptions" for metered-dose inhalers under the Montreal Protocol. J Environ Manage.2007;85:1-8.
11. D'Souza S. The Montreal Protocol and essential use exemptions. J Aerosol Med. 1995;8(suppl 1):S13-S17.
12. Food and Drug Administration. FDA News. FDA proposing phase out of CFCs in metered-dose inhalers for epinephrine. www.fda.gov/bbs/topics/NEWS
/2007/NEW01706.html. Accessed December 10, 2007.
13. Food and Drug Administration. Code of Federal Regulation 21 CFR part 2 2007N-0262. www.fda.gov/ohrms/dockets/98fr/cd0612.pdf. Accessed February 6, 2008.
14. Whitehall-Robins. RE: docket no. 98N-0148; Fed. Reg. 13258, international drug scheduling; Convention on Psychotropic Substances. April 16, 1998. www.fda.gov/ohrms/dockets/dockets/98n0148/c000010.pdf. Accessed December 30, 2007.
15. Lexi-Comp Online. www.crlonline.com. Accessed December 28, 2007.
16. Tashkin DP, Meth R, Simmons DH, Lee YE. Double-blind comparison of acute bronchial and cardiovascular effects of oral terbutaline and ephedrine. Chest. 1975;68:155-161.
17. Dulfano MJ. The new oral bronchodilators [editorial]. Chest.1975;68:133-134.
18. Cold, cough, allergy, bronchodilator, and antiasthmatic drug products for over-the-counter human use; amendment of monograph for OTC bronchodilator drug products. Fed Regist. 1996; 61:25142-25147.
19. National Association of Chain Drug Stores. Subject: use of ozone-depleting propellants; removal of essential use designation for over-the-counter epinephrine metered-dose inhalers (MDIs). January 6, 2006. www.fda.gov/ohrms/dockets/dockets/05n0374/05n-0374-EC6-Attach-1.pdf. Accessed February 6, 2008.
20. Statement of the American Pharmacists
Association (APhA) to the Food and Drug Administration's Nonprescription Drugs
Advisory Committee and the Pulmonary-Allergy Drugs Advisory Committee. Use of
ozone-depleting substance: essential-use determination of OTC epinephrine
metered dose inhalers. January 24, 2006.
www.fda.gov/ohrms/dockets/dockets/05n0374/05N-0374-EC10-Attach-1.pdf. Accessed
February 6, 2008.
To comment on this article, contact
editor@uspharmacist.com.






