US Pharm. 2022;47(10):14.
According to the FDA’s Center for Drug Evaluation and Research (CDER), a total of 430 novel drugs were approved from 2012 to 2021—an average of 43 approvals per year. This year, 24 novel drugs have been approved by the CDER to date. Novel drugs contain active moieties that have not been previously approved by the FDA as single ingredients or as part of combination products. These new molecular entities and biological products aim to serve unmet medical needs of patients and provide important innovative therapies to advance patient care.
First-in-Class and First-in-U.S. Approvals: Nearly 40% (102 of 256) of recent novel drug approvals between 2017 and 2021 were considered to be “first-in-class” by the CDER. The number of approvals for these drugs with unique mechanisms of action (compared with existing therapeutic agents) continues to increase. In 2017, 33% (15 of 46) of novel approvals were first-in-class drugs, whereas 54% (27 of 50) were designated first-in-class in 2021. Also, from 2017 through 2021, 74% (189 of 256) of novel drugs received approval in the U.S. prior to any other country. This reflects a steady percentage of “first-in-U.S.” novel approvals over the past 5 years (78% in 2017; 71% in 2018; 69% in 2019; 75% in 2020; and 76% in 2021).
First-Cycle and Orphan-Drug Approvals: Over the years, the CDER has increasingly clarified for drug developers the necessary study-design elements and other data required in drug applications to support full and comprehensive drug assessments. Since 2017, 85% or more of novel drugs each year have been approved on their first cycle of reviews. Orphan-drug approvals for rare diseases that affect fewer than 200,000 people in the U.S. have also increased, from 39% in 2017 to 52% in 2021.
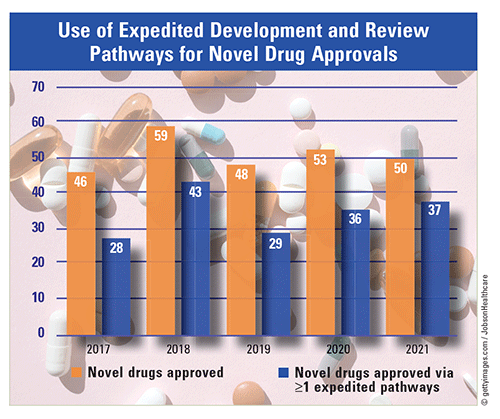
Expedited Development and Reviews: In 2021, 37 of 50 (74%) novel drugs were approved through one or more of the expedited development and review pathways: 1) fast track; 2) breakthrough; 3) priority review; and 4) accelerated approval. The use of these pathways for novel drug approvals has been been increasing overall (61% in 2017; 73% in 2018; 60% in 2019; and 68% in 2020). In particular, priority reviews and accelerated approvals have had general upward trends. Sixty-one percent of novel drugs underwent priority reviews in 2017, 73% in 2018, 58% in 2019, 57% in 2020, and 68% in 2021. Also, 13% of novel drugs underwent accelerated approval in 2017, 7% in 2018, 19% in 2019, 23% in 2020, and 28% in 2021. Notably, the CDER approved 59 novel drugs in 2018, breaking the previous record of 53 drugs in 1996.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






