US Pharm. 2013;38(4):Epub.
ABSTRACT: Opioid overdose continues to occur at staggering rates in the United States, posing numerous challenges to clinicians. The use of opioid painkillers for both medical and nonmedical purposes has increased markedly in recent years. Pharmacists in all health care venues will likely be exposed to cases of intentional and unintentional opioid overdose, and should be able to recognize the risk factors and signs of overdose, as well as understand the strategies of pharmacologic management. Furthermore, regulatory oversight of opioids is intensifying, with multiple FDA-mandated risk management programs that pharmacists should be familiar with.
Opioid overdose poses a significant public health risk in the United States, and challenges health care workers in all venues. The statistics demonstrating the growth in the occurrence of and death from opioid overdoses are staggering—drug overdose death rates in the U.S. have tripled since 1990.1 Between 1999 and 2006, the number of fatal poisonings specifically involving opioids have also tripled, with approximately 40% of all poisoning deaths being related to opioids.2 In 2008, there were 14,800 prescription painkiller deaths recorded.1 Opioids caused more overdose deaths than heroin and cocaine combined.1
The increased incidence of opioid overdoses is correlated with the widespread increase in their use. The CDC estimates that “there has been at least a 10-fold increase in the medical use of opioid painkillers during the last 20 years.”3 In 2009, pharmacies dispensed 257 million prescriptions for opioids, a 48% increase from 2000.4 Further compounding the problem is the growing use of opioids for nonmedical purposes. As described by the National Center for Injury Prevention and Control, in 2010 alone, “2 million people reported using prescription painkillers nonmedically for the first time.”1 Opioid painkillers used nonmedically were associated with approximately 300,000 emergency department visits in 2008.3 Taken together, what these statistics have equated to is an epidemic of narcotic use with severe and often fatal outcomes.
With opioid overdose serving as a prominent cause of emergency department and hospital admissions, health care pharmacists will inevitably be exposed to the opioid crisis, be it with patients at risk of experiencing overdose or with those admitted in overdose from medical or nonmedical use. It is imperative that hospital pharmacists recognize the risk factors, signs, and symptoms of an opioid overdose and be aware of the risks associated with the use of opioids, in order to position the profession to spearhead efforts to mitigate future risks to patients.
Epidemiology of Opioid Overdose
In the U.S., there are 9 million reported long-term medical users of opioids, and 5 million reported users for nonmedical use, in the past month.5 Consequently, there is an extraordinary volume of patients in whom it is crucial to identify high-risk behaviors of opioid use to help avoid dangerous overdoses. An estimated 20% of patients receiving high dosages of opioids (defined as ≥100 mg morphine equivalents per day) from one or more providers account for approximately 80% of prescription opioid overdoses.5 This statistic correlates with the trends of dosing and medical care received. According to the Office of National Drug Control Policy, from 1997-2007, “the milligram-per-person use of prescription opioids in the U.S. increased from 74 mg to 369 mg, an increase of 402%.”4
Further complicating the pharmacologic picture, opioids are commonly prescribed in conjunction with benzodiazepines and sedatives, and Paulozzi et al noted that combinations of these medications have been frequently cited in the toxicology reports of people who have died from drug overdose.6 It is important to recognize and review not only the opioid and dosage a patient is receiving, but other medications that may impair cognition or respiratory function. A number of additional risk factors have emerged through research of those most at risk of opioid overdose. Such risk factors include male sex, low income, history of substance abuse, mental illness, and geographic location in a rural area.1,3
The U.S. government has experienced increasing pressure to recognize this growing health epidemic and address the significance of these trends. In April 2011, the administration developed a plan for prescription drug abuse prevention that consists of four elements—increased education, tracking and monitoring, proper medication disposal, and enforcement.5 Educational strategies include informing the public of the risks associated with abusing opioids and educating prescribers on the safe use, storage, and disposal of these medications. Monitoring strategies include the implementation of prescription drug monitoring programs (PDMPs) in an effort reduce doctor shopping and diversion, and to improve the use of PDMPs to increase data sharing among providers. Proper medication disposal plans include the development of programs to dispose of unused drugs and enforcement plans that are focused on law enforcement strategies.4 The overarching goals of the program include reducing the abuse and misuse of opioids.
Pharmacokinetics of Opioids
Opioid analgesics are a class of opium-derived drugs that can be natural, semisynthetic, or synthetic. Opioid analgesics bind to and activate opioid receptors, referred to as the mu, kappa, and delta receptors. These are G-protein–coupled receptors distributed throughout the body, which modulate nociception as well as respiratory response, pupil constriction, and gastrointestinal (GI) motility.7-9 Prolonged exposure to opioids is thought to result in receptor desensitization. Thus, over time, larger amounts of opioids are necessary to maintain the same effects.10 However, tolerance of analgesia occurs faster than tolerance of respiratory depression, increasing the risks of respiratory depression in chronic opioid users.9
While the pharmacokinetics of the various opioids differ, the pharmacokinetic characteristics of opioid analgesics may have less of an impact in an overdose, because following an overdose there may be modified rates of absorption, variable GI motility, and drug saturation of opioid receptors.9
Opioid Overdose Symptoms and Presentation
The health care clinician should learn to recognize the most common symptoms of opioid overdose, as a patient may present in an ambulatory, community, or inpatient facility demonstrating subtle or overt clinical symptoms. A pharmacist who can recognize these symptoms may facilitate proper medical care in a timely manner for at-risk patients. Common signs to recognize in a patient with opioid overdose include respiratory depression, apnea, altered mental status, stupor, and miosis.9,11 Symptoms of opioid withdrawal include anxiety, insomnia, lacrimation, diaphoresis, mydriasis, nausea, and vomiting.11
Pharmacologic and Nonpharmacologic Management of Opioid Overdose
The primary nonpharmacologic goals in a case of opioid overdose include airway management to maintain oxygen saturation. The use of bag valve mask support or endotracheal intubation may be necessary.11 Furthermore, pharmacologic therapy for reversal may potentiate withdrawal symptoms, necessitating additional supportive care measures.
Naloxone: The key pharmacologic therapy in the acute reversal of opioid overdose is naloxone. Naloxone is a pure opioid antagonist that displaces opioids at the opioid receptor site. It is indicated for the complete or partial reversal of opioid drug effects that include respiratory depression in cases of known or suspected overdose.12 See TABLE 1 for dosing recommendations.
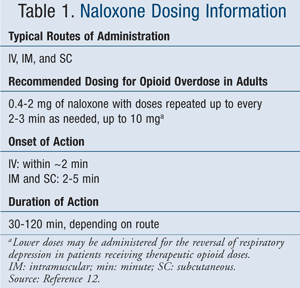
Following reversal, clinicians may need to readminister at a later interval based on the opioid ingested, as the duration of action of naloxone is limited and respiratory depression may recur.12 Continuous infusion has also been administered, as an unlabeled dosing in patients requiring several boluses to maintain respiration.11 When warranted, alternative routes of administration such as intraosseous, intranasal, inhalation by nebulization, and endotracheal have been utilized.12
Naloxone causes the release of catecholamines and, in opioid-dependent patients, may precipitate withdrawal symptoms such as hypertension, agitation, and irritability.12 Naloxone can be titrated in small increments to reverse respiratory depression while mitigating withdrawal symptoms.8 It should be used with caution in patients with cardiovascular disease and in patients with a history of seizures. Adverse reactions may occur as a result of reversing the opioid and can include severe adverse events such as cardiac arrest and ventricular fibrillation.12
Naloxone is primarily utilized in acute care facilities such as emergency departments and inpatient units, and may also facilitate postoperative opioid reversal. However, recent initiatives have explored training nonmedical personal to reverse overdose using first aid and emergency supplies of naloxone, which offers the potential for more widespread use of the drug.13,14
Pharmacologic Management of Opioid-Dependent Patients
Opioid-dependent patients who use high-dose opioids may be at risk of an overdose. Additionally, patients who experience an overdose may be encouraged to seek treatment for opioid dependence. Therapies available for the detoxification and maintenance treatment of opioid dependence include methadone and buprenorphine. Pharmacists should have familiarity with the pharmacologic properties of these medications, as well as with the prescribing restrictions associated with these drugs.
Methadone is a full mu-receptor agonist that is indicated for the detoxification and maintenance treatment of opioid dependence.10 It is a Schedule II controlled substance that is also indicated for the treatment of moderate-to-severe pain. Methadone utilized for opioid dependence is subject to the regulations outlined by the Substance Abuse and Mental Health Services Administration’s (SAMHSA) Center for Substance Abuse Treatment (CSAT), and further regulations for the use of methadone may vary by state.15 Additionally, methadone has an associated Risk Evaluation and Mitigation Strategy (REMS) program. Methadone is titrated to a maintenance dose to mitigate addictive cravings and may cause adverse events, including central nervous system (CNS) depression, hypotension, QT prolongation, and respiratory depression.15
Buprenorphine is a partial mu agonist and kappa antagonist that is indicated as maintenance therapy for opioid dependence.10,16 Buprenorphine is a Schedule III controlled substance that is also indicated for moderate-to-severe acute or chronic pain. Prescribers must meet special qualification criteria and have a DEA number specific to prescribing buprenorphine in order to prescribe the drug for opioid dependence. Additionally, buprenorphine has an associated REMS program.16 Buprenorphine is also available in combination with naloxone.10,16 Adverse effects to monitor for include CNS depression, hypotension, QT prolongation, hypersensitivity reactions, hepatitis, and respiratory depression.10,16
Regulatory Oversight and Opioid REMS
The government has addressed the growing issue of opioid-related public health and safety issues, including addiction, overdose, and death. As a part of the federal initiatives aimed at reducing prescription drug abuse, misuse, and overdose, REMS have been developed for a number of opioid agents.17
As a result of the FDA Amendments Act of 2007, the FDA has the authority to enforce postmarketing risk management strategies. Thus, the FDA is now mandating that manufacturers develop REMS for specific types and classes of drugs that it has deemed necessary in order to have strategies in place to ensure that the benefits of use outweigh the risk of the medication.18 Opioid REMS programs are designed to mitigate opioid drug misuse and abuse and improve safe utilization of opioids, while continuing to enable access to such medications for patients in need of opioids for medical use.19
Previously, a number of opioid analgesics had implemented drug-specific REMS programs with individual, manufacturer-specified programs for each drug. In an effort to consolidate and simplify the REMS, the FDA has implemented two classwide REMS programs for transmucosal fentanyl products—the Transmucosal Immediate-Release Fentanyl (TIRF) REMS Access Program (March 2012) and Extended-Release and Long-Acting (ER/LA) Opioid Analgesics REMS (July 2012).19,20
TIRF REMS: The TIRF REMS Access Program was approved as a shared system for all transmucosal fentanyl products and has replaced the individual REMS programs for these medications. The TIRF program enables pharmacies and prescribers a single enrollment into the system in an effort to reduce the burden of the REMS program. TIRF medications (TABLE 2) are indicated for opioid-tolerant patients for the use of cancer pain.20
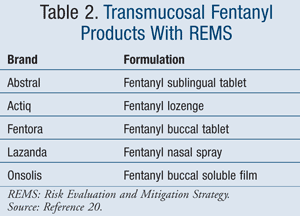
Importantly, health care providers who prescribe TIRF medications for use only within the inpatient settings of hospitals, hospice, or long-term care facilities are not required to enroll in the TIRF REMS Access Program. However, both inpatient and outpatient pharmacies are required to enroll in the program. Patients prescribed TIRF medications as an outpatient are required to sign a patient-prescriber agreement and are provided with a medication guide. Prescriptions must be filled at an enrolled pharmacy.20
ER/LA REMS: ER/LA opioids were also identified as a subset of opioids at high risk of abuse and misuse, while simultaneously having a high volume of use; in 2011, 22.9 million prescriptions were dispensed for ER/LA opioids.17 Consequently, a classwide REMS was introduced for all ER/LA opioid products (TABLE 3).21,22 The components of the ER/LA REMS include the FDA blueprint for prescriber education, updated medication guides and patient counseling documents, and FDA assessment and auditing of drug manufacturers.17
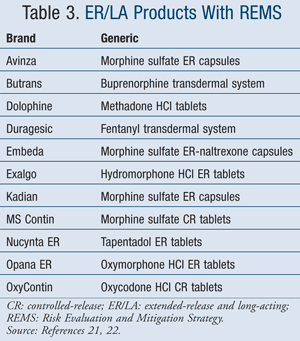
In July 2012, the Blueprint for Prescriber Education for Extended-Release and Long-Acting Opioid Analgesics was released by the FDA.21 The goals of the prescriber education include understanding how to assess patients for therapy; how to initiate, modify, and discontinue therapy; how to counsel patients and caregivers about the use, storage, and disposal of their opioid medications; and how to understand ER/LA-specific concerns, including the risks associated with overdose due to the long-acting formulations.21
REMS continuing education (CE) activities are commencing in 2013, with the first CE programs available as of March (a list of these programs is available at www.ER-LA-opioidREMS.com).22 While the training is currently not mandatory for prescribers and not a requisite for prescribing ER/LA opioids,17 the expectation of the FDA is that drug manufacturers will train at least 60% of the 320,000 prescribers of ER/LA within 4 years.22
Joint Commission Sentinel Event Alert: Further underscoring the widespread concern for opioid-related adverse events, in August 2012 the Joint Commission issued a Sentinel Event Alert focused on the safe use of opioids in hospitals.23 The alert noted that 16% of inpatient adverse drug reactions in a recent study were related to opioids. Reports from the Sentinel Event database (2004-2011) demonstrated that 47% of opioid-related adverse drug events were attributable to medication errors related to the wrong dose, and 29% were attributable to patient monitoring.23
The alert also stated that opioid-induced respiratory depression is of notable concern in hospitalized patients, particularly in vulnerable patient populations such as those receiving higher doses of opioids, those with concurrent risk factors such as sleep apnea, those receiving interacting medications including other CNS and respiratory depressants, and the young or elderly.23
Role of the Pharmacist in Opioid Overdose
Pharmacists in all venues of health care and hospital pharmacy can play a crucial role in identifying patients at risk, reviewing medication profiles, monitoring patients, educating prescribers, and providing counseling on medications to heighten awareness of the risks of drug interactions and the overdose of opioids. Pharmacists may be involved in the following ways:
- Review patient profiles or perform a patient interview to assess a patient’s history of opioid use and abuse, risk of respiratory depression, concurrent medications the patient is taking, and whether or not the patient is opioid naïve.
- Review each medication profile to identify concurrent drugs such as additional opioids, sedatives, or benzodiazepines.
- Ensure that the prescriber is aware of all medications the patient has been receiving at home, and confirm that medication reconciliation is complete and all dosages are recorded.
- If opioids are being converted from one morphine equivalent to another, ensure that the lowest starting dose is initiated and carefully and slowly up-titrated only after proper patient monitoring.
- Review dose conversions, particularly when the drug or route is being modified.
The Joint Commission Sentinel Event Alert emphasizes the importance of monitoring patients at risk of respiratory depression. This monitoring includes physical assessment and physiological monitoring.23 Pharmacists in health care facilities may be involved in creating or improving policies and procedures related to opioid prescribing and monitoring or to the reporting of adverse drug events. Pharmacists can have an important impact by analyzing reported adverse drug events to assess for opportunities for patient safety improvement strategies. The implementation of dose limits and alerts for opioid dosing can be supported by utilizing informatics programs, including computerized prescriber order entry (CPOE). These tools may also by utilized to aid clinicians in the conversion of opioid dosages.
Additionally, pharmacists should take responsibility for furthering the education of clinicians and patients alike on risk factors, common risk points, and safety mitigation strategies. Counseling patients in the hospital or ambulatory setting on new medications or on discharge medications, particularly on the REMS opioids such as fentanyl products and long-acting opioids, is another opportunity to help reinforce patient education. Pharmacists in hospital and health system pharmacy have an important opportunity and responsibility in mitigating overdoses and ensuring that safety strategies are in place relating to the use of opioids.
REFERENCES
1. Policy impact: prescription painkiller overdoses. CDC.
November 29, 2012. www.cdc.gov/homeandrecreationalsafety/rxbrief/
Accessed December 15, 2012.
2. Warner M, Chen LH, Makuk D. Increase in Fatal Poisonings Involving Opioid Analgesics in the United States, 1999-2006. NCHS Data Brief No. 22. Hyattsville, MD: National Center for Health Statistics; 2009.
3. Injury and violence prevention and control. Unintentional drug poisoning in the United
States. CDC. July 2010. www.cdc.gov/homeandrecreationalsafety/pdf/poison-issue-brief.pdf. Accessed December 15, 2012.
4. Prescription drug abuse. Office of National Drug
Control Policy. www.whitehouse.gov/ondcp/prescription-drug-abuse.
Accessed December 19, 2012.
5. CDC. CDC grand rounds: prescription drug overdoses—a U.S. epidemic. Morb Mortal Wkly Rep MMWR. 2012;61:10-13.
6. Paulozzi LJ, Weisler RH, Patkar AA. A national epidemic
of unintentional prescription opioid overdose deaths: how physicians
can help control it. J Clin Psychiatry. 2011;72:589-592.
7. McDonald J, Lambert DG. Opioid receptors. CE Anaesth Criti Care Pain. 2005;5:22-25.
8. Mace SE, Ducharme J, Murphy MF. Pain Management and Sedation: Emergency Department. New York, NY: McGraw-Hill Companies, Inc; 2006.
9. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367:145-155.
10. Boothby LA, Doering PL. Buprenorphine for the treatment of opioid dependence. Am J Health Syst Pharm. 2007;64:266-272.
11. Glaspy J. Opioids. In: Ma O, Cline D, Tittinalli J, et al, eds. Emergency Medicine Manual. 6th ed.
New York, NY: McGraw-Hill Companies, Inc; 2004.
www.r2library.com/resource/detail/0071410252#section=ch0103s1313.
Accessed December 28, 2012.
12. Lexicomp Online. Naloxone. www.crlonline.com. Accessed December 13, 2012.
13. Belestsky L, Rich J, Walley A. Prevention of fatal opioid overdose. JAMA. 2012;308:1863-1864.
14. CDC. Community-based opioid overdose prevention programs providing naloxone—United States, 2010. Morb Mortal Wkly Rep MMWR. 2012;61:101-105.
15. Lexicomp Online. Methadone. www.crlonline.com. Accessed December 13, 2012.
16. Lexicomp Online. Buprenorphine. www.crlonline.com. Accessed December 13, 2012.
17. FDA introduces new safety measures for
extended-release and long-acting opioid medications. FDA. July 9, 2012.
www.fda.gov/newsevents/newsroom/pressannouncements/ucm310870.htm.
Accessed October 18, 2012.
18. Approved Risk Evaluation and Mitigation Strategies
(REMS). FDA.
www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm111350.htm#information.
Accessed October 18, 2012.
19. Risk Evaluation and Mitigation Strategy (REMS) for
extended-release and long-acting opioids. FDA. July 9, 2012.
www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm163647.htm.
Accessed October 18, 2012.
20. FDA approves shared system REMS for TIRF products.
FDA. December 29, 2012.
www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm285345.htm.
Accessed October 18, 2012.
21. Introduction for the FDA blueprint for prescriber
education for extended-release and long-acting opioid analgesics. FDA.
July 9, 2012.
www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrugClass/UCM277916.pdf.
Accessed October 18, 2012.
22. Questions and answers: FDA approves a Risk Evaluation
and Mitigation Strategy (REMS) for extended-release and long-acting
(ER/LA) opioid analgesics. FDA. March 1, 2013.
www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm309742.htm.
Accessed March 17, 2013.
23. The Joint Commission. Safe use of opioids in hospitals. The Joint Commission Sentinel Event Alert. 2012;49:1-5. www.jointcommission.org/assets/1/18/SEA_49_opioids_8_2_12_final.pdf. Accessed December 1, 2012.
To comment on this article, contact rdavidson@uspharmacist.com.





