US Pharm.
2006;31(9):50-71.
Pregnant
women represent a challenging population to treat with both OTC and
prescription medications. Well-designed, prospective studies are seldom
conducted in pregnant women, and the safety and efficacy of drug therapy is
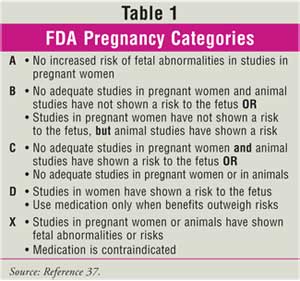
often unclear (Table 1). Therefore, health care practitioners have only
modest guidance on what medications are safe for use in pregnant women.
Pharmacists face this dilemma on a daily basis with regard to OTC medications
during pregnancy. Herein, the appropriate treatment of six gastrointestinal
(GI)disorders that occur during pregnancy will be reviewed.
Nausea and Vomiting
Nausea and vomiting
of pregnancy (NVP) most commonly affects women in their first trimester. The
frequency of NVP may be variable as up to 85% and 50% of pregnant women
experience nausea and vomiting, respectively.1 Although often
referred to as morning sickness, NVP can occur at any time during the day. The
etiology of NVP is unknown and controversial. Psychological factors, GI tract
dysfunction, and hormonal changes have been studied as possible causes, but
the data are inconclusive. Another study has shown that chronic Helicobacter
pylori infection was significantly more prevalent in pregnant women suffering
from NVP, compared to pregnant women not suffering from NVP.2
Nonpharmacologic Treatment
Pharmacists should
not recommend pharmacologic treatment for NVP. For mild cases of NVP,
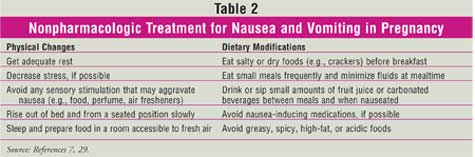
nonpharmacologic methods to prevent nausea (Table 2) can be
recommended. However, pregnant women should always be referred to their
physician if such measures are ineffective or if NVP symptoms are more severe.
Several serious conditions can initially present with moderate to severe
nausea and vomiting. These conditions range from preeclampsia to liver
disorders. If a pregnant patient presents with moderate to severe nausea and
vomiting, she should be referred to her physician for further evaluation.
It is important to assess the
appropriateness of physician recommendations. For nonpharmacologic therapy,
physicians may recommend acupressure. There are conflicting data on the
effectiveness of acupressure bands. However, these devices appear to be safe.
Pharmacologic Treatment
The American
College of Obstetrics and Gynecology (ACOG) issued recommendations for the
treatment of nausea and vomiting in pregnancy in 2004.3 One
recommendation is for a woman to take a multivitamin while she is trying to
conceive. It has been noted in clinical trials that there may be a lower
incidence of vomiting if women take a multivitamin at the time of conception.
3 Physicians may suggest many drug therapy options to treat NVP,
including pyridoxine, antiemetics, antihistamines, corticosteroids,
antimotility agents, and anticholinergics. In general, drug therapy is
typically reserved for severe cases of NVP after balancing risk versus benefit.
Ginger, on the other hand, has
been shown to decrease nausea and vomiting in cases of hyperemesis gravidarum,
and there are no reports of fetal abnormalities.4,5 Ginger contains
gingerols and shogaols that may directly affect the digestive tract to prevent
nausea and vomiting. Yet, the use of ginger remains controversial. Some warn
against the use of ginger because of possible antiplatelet effects. Ginger
contains thromboxane synthetase inhibitor. This inhibitor may interfere with
testosterone receptor binding in the fetus.6 However, ginger is
used as a spice in other cultures in amounts similar to those used to treat
NVP, and there are no reports of harm caused with these doses.7
Ginger is available in many forms (e.g., ginger ale, ginger root, and
tablets). Administration of 1 g/day in divided doses before meals and at
bedtime has been used to prevent nausea.
Emetrol:
Emetrol, a combination of dextrose, levulose, and phosphoric acid, is used
off-label for treating NVP. This combination of ingredients may act directly
on the GI tract to decrease smooth muscle contraction, thus delaying gastric
emptying. The pregnancy category for Emetrol is unknown. Table 1 lists
pregnancy categories for all medications discussed in this article.
Pyridoxine:
Pyridoxine (vitamin B6), either alone or combined with doxylamine,
has been used to prevent nausea and vomiting. ACOG recommends pyridoxine as
the first step in a pharmacologic treatment plan, due to available safety and
efficacy data. Pyridoxine is pregnancy category A if used at doses comparable
to the recommended daily allowance (RDA) of 2.2 mg. In fact, many pregnant
women may be vitamin B6 deficient, since the demand for this
vitamin increases during pregnancy. Exceeding the RDA is not recommended,
because the pregnancy category falls to a C level at such doses. The
recommended dose of pyridoxine as a treatment for nausea and vomiting ranges
from 10 to 25 mg three to four times daily. The prenatal vitamin PremesisRx
contains 75 mg of pyridoxine. While this combination makes theoretical sense,
this product has not been proved to reduce NVP.3
Pyridoxine was once combined
with doxylamine in a product known as Bendectin, which was withdrawn from the
market in 1983 due to possible birth defects. However, evidence indicates that
this combination may not be teratogenic. Furthermore, doxylamine is pregnancy
category B. In 1983, the FDA regarded three studies as the most conclusive
regarding the teratogenicity of Bendectin but concluded that there was no
definite causal relationship, since nausea and vomiting could increase the
risk of teratogenicity. It was not possible to determine if use of Bendectin
was completely devoid of fetal risk. Bendectin was thus withdrawn from the
market to avoid possible litigation. Pyridoxine-doxylamine (Diclectin) is
available in Canada and is still recommended by U.S. physicians. The standard
dose of doxylamine is 12.5 mg three to four times daily. Doxylamine is
recommended for use in combination with pyridoxine for patients in whom
pyridoxine monotherapy was not effective.3
Anticholinergic
Medications: Women
may also be advised to take antihistamines or anticholinergics such as
meclizine, dimenhydrinate, and diphenhydramine. These medications are used to
treat NVP, due to their effectiveness. All three are rated pregnancy category
B and have not been associated with an increased risk of malformations.3
Common doses are diphenhydramine 25 to 50 mg every four to six hours as
needed or dimenhydrinate 50 to 100 mg every four to six hours as needed.
In summary, no OTC medications
are FDA approved for the treatment of NVP. For women considering becoming
pregnant, a multivitamin should be recommended. If a pregnant patient is
experiencing nausea and/or vomiting, nonpharmacologic treatment can be
suggested as initial therapy. However, if this fails to control symptoms, or
if a woman has moderate to severe nausea and vomiting, she should be referred
to her physician for further evaluation. If an OTC medication is recommended,
it should be used in conjunction with nonpharmacologic measures. Pharmacists
should counsel patients to take the medication on an "as-needed" basis,
rehydrate if necessary, and follow up with their physicians if nausea and/or
vomiting worsens.
GERD/Heartburn
Heartburn or
gastroesophageal reflux disease (GERD) is common during pregnancy and is
reported by about 30% to 50% of pregnant women; the incidence may be as high
as 80% in certain populations.8-10 Although the exact mechanism is
unclear, reduced lower esophageal sphincter (LES) pressure has a role in the
pathogenesis of GERD in pregnancy. During early pregnancy, the LES pressure
responses to hormonal, pharmacologic, and physiologic stimulation are reduced.
11 The resting LES pressure falls about 33% to 50% of baseline, probably
due to increased progesterone levels.8 Abnormal gastric emptying or
delayed small bowel transit may also contribute to the pathogenesis, but the
impact of increased abdominal pressure due to an enlarging uterus is
controversial.8
Symptoms
GERD symptoms
during pregnancy are similar to those in the general population.8,12
Heartburn is the predominant symptom, but regurgitation also occurs
frequently.8 Symptoms can occur at any time during pregnancy but
are generally worse during the last trimester.9,13 Dietary triggers
that worsen symptoms include fatty foods, spicy foods, and caffeine. Since
GERD during pregnancy usually has a short duration and resolves after
delivery, complications (e.g., esophagitis and stricture) are rare.8

Nonpharmacologic Treatment
The preferred and
safest initial treatment for GERD during pregnancy is lifestyle modification.
Such measures include avoiding foods that trigger symptoms (e.g., spicy or
fatty food) and elevating the head of the bed (see Table 3). Chewing
gum may also help because it stimulates salivary glands, which can help
neutralize acid.8 Lifestyle modifications may control mild symptoms
in women, but drug therapy may be necessary if symptoms are not relieved by
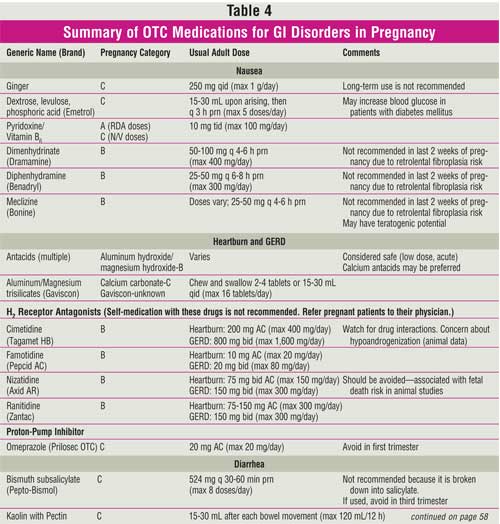
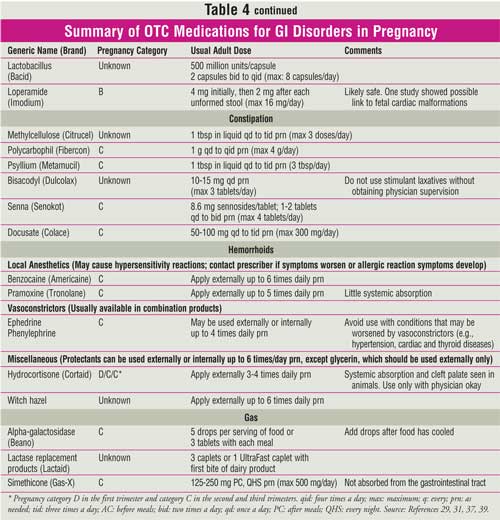
these measures (Table 4).
Antacids
The FDA has not
determined the pregnancy category for antacids, and there are few data on the
effects of antacids on the fetus. In animal studies, magnesium-, aluminum-, or
calcium-based antacids were not teratotogenic.14 One retrospective
case-controlled study reported a significant increase in congenital
malformations in infants with third trimester antacid exposure. However,
analysis of individual antacids found no link to congenital anomalies.15
Antacid use has not been shown to be unsafe, but there have been no
controlled studies.16
Despite the limited data, experts
have agreed that antacids should be the first OTC treatment for heartburn
during pregnancy.12 Antacids provide fast and effective relief, and
up to half of women will require only antacids to control GERD.8
Magnesium-, calcium-, and aluminum-containing antacids at recommended doses
are considered safe to use during pregnancy, although some experts
preferentially recommend calcium/magnesium-containing antacids, because high
doses of aluminum-containing antacids may increase aluminum levels in women
and cause fetal harm.8,12,17
Alginates are generally as
effective as antacids in controlling GERD symptoms.18 A formulation
of Gaviscon that has a lower sodium content was studied in an open-label study
in 150 pregnant women.19 Although effective in relieving heartburn,
adverse events were reported in 10 fetuses.19 Furthermore,
magnesium trisilicate (found in some Gaviscon preparations) may cause fetal
nephrolithiasis, hypotonia, respiratory distress, and cardiovascular
impairment if used long-term and at high doses.19
In summary, calcium- or
magnesium-containing antacids are preferred during pregnancy. Usual dosage of
antacids on an as-needed basis should be recommended. Calcium-based antacids
provide an additional source of calcium during pregnancy, which may help
prevent hypertension and preeclampsia.12 Patients should be
counseled on potential interactions of antacids with other drugs and
supplements such as iron.
Histamine-2 Receptor Antagonists
(H2RAs)
All H2
RAs are FDA pregnancy category B. Cimetidine and ranitidine have been used
safely during pregnancy over the last 30 years.8 Only ranitidine,
however, has been specifically evaluated in heartburn during pregnancy.
Both studies, albeit small and short in duration, showed improvement in
heartburn symptoms with ranitidine and no adverse pregnancy outcomes.9,19
There are no prospective randomized studies on the safety of cimetidine and
other H2RAs during pregnancy, but the general consensus is that
cimetidine and possibly famotidine are safe.8,12 Cohort studies
have shown similar pregnancy outcomes and rates of malformation in women
exposed to H2RAs during the first trimester, compared to controls.
20,21 Although one animal study suggested antiandrogenic effects of
cimetidine and feminization of male offspring, data are conflicting and have
not been reported in humans.8,16,22 Famotidine was not fetally
toxic or teratogenic in animal studies. Animal studies of nizatidine have
conflicting results. One showed no adverse effects on fetal rat pups, but
another study in rabbits given 300 times the recommended dose for humans
resulted in abortions, low fetal weights, and fewer live fetuses. Thus, other H
2RAs may be safer than nizatidine.
For women with refractory
symptoms despite lifestyle modification and antacid use, H2RAs are
commonly used for pregnant women.12 Ranitidine is preferred due to
available data, but cimetidine is likely safe. If symptoms persist with
antacids, physician referral is advised. A pregnant woman should use the
lowest dose necessary (e.g., 75 mg of ranitidine once daily) until she is
evaluated by a physician.
Proton-Pump Inhibitors
(PPIs)
Omeprazole, the
only PPI available OTC, is pregnancy category C. Recent retrospective and
prospective cohort studies suggest that usual doses of omeprazole and other
PPIs during the first trimester do not present a major teratogenic risk in
humans.23-26 A prospective cohort study was conducted on the safety
of PPI use in pregnancy.27 In 410 pregnant women taking PPIs (295,
omeprazole; 62, lansoprazole; 53, pantoprazole) mostly in the first trimester,
the rate of major anomalies was comparable between PPI-exposed women and
controls. Although these data suggest safety of use, PPIs should be used
during pregnancy with medical supervision and only in women with complicated
GERD refractory to other therapies.8,12 These women should be
referred to a medical practitioner for further evaluation and management.
In summary, a pregnant woman
complaining of GERD or heartburn can be instructed to use nonpharmacologic
treatment. Antacid therapy may be recommended if lifestyle modification does
not control symptoms. If symptoms continue, low-dose H2RA therapy
can be used until she can be seen and evaluated by a physician. Omeprazole OTC
should be reserved for patients who are acting on the recommendation of their
physician.
Diarrhea
Diarrhea is
characterized by an increased frequency and decreased consistency of fecal
discharge, compared to a normal bowel movement pattern.28 More than
three bowel movements per day is considered abnormal.29 Diarrhea is
further classified as acute or chronic, based on its onset and duration. Four
mechanisms lead to diarrhea: a change in active ion transport by decreased
sodium absorption or increased chloride secretion, change in intestinal
motility, an increase in luminal osmolarity, and an increase in tissue
hydrostatic pressure.28 Diarrhea in pregnancy is typically linked
to viral or bacterial infection.30 About 80% of diarrhea episodes
are due to viral infection.31 The exact epidemiology of diarrhea in
pregnancy is unknown.28 Complications of diarrhea include
electrolyte imbalance and dehydration.7
Symptoms
Diarrhea symptoms
in pregnancy are similar to those seen in the general population. Patients
with acute diarrhea present with increased bowel movements. Acute diarrhea may
also present with abrupt onset of nausea, vomiting, abdominal pain, headache,
fever, chills, and malaise. Stools have a decreased consistency but are never
bloody. It generally lasts 12 to 60 hours. When assessing patients with
diarrhea, it is important to inquire about the frequency, volume, consistency,
and color of stools.28
Nonpharmacologic Treatment
The first step in
treating diarrhea is rehydration of fluids and electrolytes. Oral replacement
therapy (ORT) can be used to treat nearly all cases of diarrhea. The World
Health Organization recommends oral rehydration solutions that take advantage
of the sodium/glucose couples active absorption mechanism. These solutions
contain sodium and glucose and are formulated with a concentration and
osmolality similar to luminal fluid.30 ORT options include
decaffeinated beverages and juices. Sorbitol-containing ORTs should be
avoided, as they may worsen preexisting diarrhea. Patients should also be
advised to minimize or avoid caffeine, alcohol, and sugary beverage intake,
because these products may also worsen preexisting diarrhea. If the patient
has worsening diarrhea or is unable to keep up her diarrhea losses, she should
be referred to her physician. If the patient has stupor, coma, intestinal
ileus, or protracted vomiting, she should be taken to the emergency department
for further evaluation.30 Additional questions to ask to determine
whether OTC diarrhea treatment or physician referral is necessary are shown in
Table 5.
Opiates and Opiate
Derivatives
Opiate and opioid
derivatives work by delaying the transit of intraluminal content or by
increasing gut capacity, prolonging contact and absorption. Most opiates work
peripherally and centrally. Loperamide, the only opiate derivative available
OTC, is the exception and works peripherally. It has antisecretory effects and
works by inhibiting the calcium-binding protein calmodulin and by controlling
chloride secretion.28 Loperamide is pregnancy category B, and its
use in pregnancy has not been associated with major malformations.30,32
However, one study showed that there may be a link between loper amide
use and fetal cardiac malformation.31
Adsorbents
Adsorbents such as
attapulgite, kaolin, polycarbophil, and pectin are used for symptomatic relief
in mild diarrhea. There is little evidence of their efficacy, and they may not
alter stool frequency, stool fluid losses, or the duration of diarrhea.
Adsorbents have a nonspecific mechanism of action. These compounds may adsorb
nutrients, toxins, drugs, bacteria, and digestive enzymes in the GI tract.
28 According to the FDA, polycarbophil is the only effective adsorbent
agent. Polycarbophil may modify watery stool output by absorbing up to 60
times its weight in water.29 Adverse effects of adsorbents are
constipation, bloating, and fullness.28 These products are
pregnancy category B.30

Bismuth Subsalicylate
Bismuth
subsalicylate has antisecretory, anti-inflammatory, and antibacterial effects.
28 It works by reacting with hydrochloric acid in the stomach to form
bismuth oxychloride and salicylic acid.29 It reduces the frequency
of unformed stools, increases stool consistency, and relieves symptoms of
abdominal cramping, nausea, and vomiting.28 Bismuth subsalicylate
is pregnancy category C.30 Similar to other salicylate products,
bismuth subsalicylate should not be used in the second and third trimesters
due to risks to the mother and fetus. These risks include premature closure of
the ductus arteriosus, bleeding, and potential for increased perinatal
mortality.33 This product should be used only under the supervision
of the patient's physician.31
Digestive Enzymes
Lactobacillus
acidophilus is a
digestive enzyme that helps shorten the course of diarrhea by restoring the
normal flora. It may prevent changes in fecal flora and the resulting diarrhea
associated with antibiotics.28 Limited data suggest no link between
lactobacillus use and congenital abnormalities, but further research is needed.
34
A
variety of OTC products are available to treat diarrhea. Many are pregnancy
category B; however, pregnant women should consult their physician prior to
using any OTC medications for diarrhea. For pregnant patients with mild
diarrhea and minimal dehydration, it is safe to recommend ORT.
Constipation
Constipation
affects 11% to 38% of pregnancies.35 As in GERD, increasing
progesterone levels have been implicated in the pathogenesis of constipation.
Progesterone can decrease GI motility and may inhibit motilin, which also
affects GI transit time.33 The effects of progesterone combined
with decreased GI motility can cause constipation during pregnancy. Other
predisposing factors for constipation include iron supplementation and an
enlarging gravid uterus.33 The symptoms of constipation that a
pregnant patient experiences are comparable to those of nonpregnant adults.
These include straining, feelings of incomplete evacuation, and changes in
stool frequency and/or consistency.12 If a pregnant woman develops
abdominal pain, nausea, vomiting, weight loss, rectal bleeding, or cramping
with constipation, she should be referred to a physician immediately.29
Nonpharmacologic Treatment
Lifestyle
modification is the preferred and safest initial treatment for constipation
during pregnancy. Modifications include increasing dietary fiber, liquid
intake, and physical activity. Fiber therapy has been shown to increase stool
frequency and soften formed stools.35 Yet, there are conflicting
data on fiber's efficacy in treating constipation. The type of fiber may be
one reason for this discrepancy. Some types of fiber, such as bran, are poorly
absorbed. This type is better able to absorb water and is therefore more
effective. Sources of fiber include pharmacologic or dietary supplementation.
Supplementation should be gradual to lessen bloating and gas. Surprisingly,
increasing fluid intake is also controversial. Increased fluids work best for
constipated patients who are dehydrated.36 Liquid intake may be
compromised by nausea and vomiting, especially in the first trimester.
Minimizing the intake of foods that may lead to constipation, such as bananas,
cabbage, or beans, may also be beneficial. While controversial, lifestyle and
dietary modifications may be sufficient to control mild constipation.
Physician referral and drug therapy may be necessary if symptoms are not
relieved by these measures (Table 6).

Bulk-Forming Laxatives
Bulk-forming
laxatives are advised for use in pregnancy, but most are pregnancy category C.
This recommendation is based on their limited systemic absorption and good
safety profile. Three common bulk-forming laxatives are available for general
use: methylcellulose, polycarbophil, and psyllium. These agents work by
increasing the water contained in stool, which improves stool weight and
consistency.33 The bulkier stool, in turn, stimulates peristalsis.
Paradoxically, the increase in stool consistency is also the reason why these
agents can be used for the treatment of diarrhea. Bulk-forming agents can
cause flatulence and/or bloating, which may already be a problem for this
patient population. These adverse effects can be minimized by increasing fluid
intake and by careful dose titration. Increased fluid intake will also
decrease esophageal irritation/obstruction. Psyllium has been associated with
allergic reactions, including anaphylaxis.12 Common doses are
listed in Table 4.
Docusate
Docusate works
differently than other laxatives. Docusate decreases stool surface tension,
which increases the fluid content of the stool, thereby softening stools.
33 Docusate is pregnancy category C but undergoes minimal systemic
absorption and has not been associated with congenital defects.34,37
To relieve possible constipation with prenatal vitamins, some vitamins also
contain docusate. This is an appropriate option after a patient has not
achieved relief using nonpharmacologic measures and bulk-forming laxatives.
Stimulant Laxatives
Stimulant laxatives
may be reserved for patients whose symptoms do not improve with
nonpharmacologic treatment and bulk-forming laxatives or docusate. Their use
should be infrequent, not habitual. The most common stimulant laxatives are
bisacodyl and senna, which are pregnancy category C. These products act on the
colon to stimulate motility. It is this mechanism of action that predisposes
them to a higher incidence of diarrhea and abdominal pain than with
bulk-forming laxatives.35 Common doses are 5 to 15 mg of bisacodyl
orally per day or 10 mg suppository rectally per day and 1 to 2 tablets of
senna orally twice daily. Senna has not been shown to be teratogenic in
animals or humans.33,34,37
Numerous laxatives are available OTC, but
they should be used sparingly. Mineral oil should be avoided, since it can
decrease vitamin absorption and may increase the risk of lipid pneumonia.
29,33,38,39 Castor oil may cause uterine contractions and should not be
used in pregnancy.33,38 Magnesium and saline laxatives are not
recommended in pregnancy because of their potential effects on the patient's
electrolyte balance and ability of the latter to cause water retention.
29,33,38 The potential for adverse events due to osmotic laxatives is
higher in patients with preexisting renal disease or heart failure.
In summary, constipation during pregnancy
should first be treated with nonpharmacologic therapy. Then, products that are
not systemically absorbed should be used, such as bulk-forming laxatives.
29,30 Stimulant laxatives are second-line therapy and are recommended
only if increased dietary fiber and bulk-forming laxatives fail to relieve
constipation. Consultation with a physician is recommended prior to using
pharmacologic agents.
Hemorrhoids
Hemorrhoids have been frequently
reported in the second and third trimesters of pregnancy. Although the
etiology of hemorrhoids is unclear, several factors involved in the
pathogenesis are well known. These factors include preexisting bowel disease,
a low-fiber diet, straining with defecation, and vascular engorgement due to
increased pressure caused by the enlarging abdomen.33
Symptoms
Hemorrhoids can
present with symptoms similar to other anorectal disorders. The pharmacist
should verify that the patient has had her symptoms evaluated by a physician
before recommending a therapy. Swelling, itching, and burning are common
symptoms and are similar to symptoms seen in nonpregnant adults.40
Before making any recommendations, the severity of the patient's symptoms
should be assessed. Pain, severe symptoms, bleeding, fecal seepage, rectal
protrusion, bowel habit changes, and a family history of colon cancer all
merit immediate physician evaluation.29
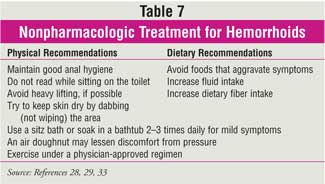
Nonpharmacologic Treatment
As with other GI
complaints, hemorrhoids should be treated nonpharmacologically with increasing
dietary liquid and fiber. Foods and beverages that cause or aggravate symptoms
should be restricted. Soaking in warm water in a sitz bath or a bathtub may
also provide relief. Due to the relationship between straining and
constipation and hemorrhoids, many treatments for constipation are also
appropriate for hemorrhoids. Avoiding constipation and contributing factors to
constipation will help improve symptoms. Nonpharmacologic measures that can
provide relief are summarized in Table 7.
Pharmacologic Treatment
Local anesthetics,
vasoconstrictors, topical corticosteroids, protectants, and astringents may be
used to treat hemorrhoids. Topical anesthetics help relieve itching, burning,
and pain.33 Absorption is minimal unless the skin is chafed. These
products should be applied externally to the skin, and the patient should be
counseled to watch for allergic reactions, which may mimic their hemorrhoid
symptoms. Some vasoconstrictors are ephedrine, epinephrine, and phenylephrine.
These agents decrease swelling but should not be used in pregnant women who
have medical conditions that may be exacerbated by vasoconstriction, such as
hypertension. It is difficult to assess the safety of vasoconstrictors in
pregnancy because they are usually available in combination products. Topical
hydrocortisone is another option for pain and itching relief. Several
protectant products (e.g., petrolatum) are available and prevent further skin
damage by providing a barrier against moisture. Petrolatum has poor absorption
and is considered safe in pregnancy.29 Astringents such as witch
hazel can sting, which may also exacerbate underlying symptoms. These topical
agents have not been adequately evaluated for safety and efficacy in pregnant
patients. Of all of these options, topical anesthetics, astringents, and skin
protectants are likely the most safe. However, due to limited safety data,
these products should be used under a physician's supervision.
Conservative management for hemorrhoids is
aimed at pain relief and stool softening. Other options for external
hemorrhoids are topical anesthetics, vasoconstrictors, protectants,
astringents, and topical corticosteroids. For pain relief, acetaminophen
(pregnancy category B) can be recommended. NSAIDs should be avoided, as they
may worsen bleeding and are pregnancy category D in the third trimester.
Low-strength corticosteroid creams and/or witch hazel can be used for itching.
33 If used, topical corticosteroids should be applied in the smallest
amount and for the shortest duration possible. Docusate can also be used to
soften stools. Due to the lack of safety information regarding these products
in pregnant patients and potential hemorrhoid complications that may be masked
by inappropriate treatment, a physician should be consulted prior to
recommending OTC products for hemorrhoid treatment.29
Conservative options are recommended to
treat hemorrhoids during pregnancy. Since constipation may exacerbate
hemorrhoids, prevention and treatment of constipation is also recommended. A
physician should be consulted to evaluate other options if pain is not
relieved by conservative methods. The patient should contact her physician if
symptoms worsen, persist for more than seven days, or if hemorrhoids are
linked to severe pain, protrusion, bleeding, or fecal seepage.29
GI Gas
GI gas manifests in
two forms: belching and flatulence. Belching, also known as eructation,
is the voluntary or involuntary release of gas from the esophagus or stomach.
Belching mainly occurs after a meal, due to the relaxation of the LES. Most of
the gas in the stomach comes from swallowing air. When too much air is
swallowed, an individual may experience flatulence or abdominal pain, as well
as belching. Chewing gum, eating rapidly, smoking, and drinking carbonated
beverages may contribute to excessive ingestion of air. Flatulence occurs due
to gas trapped in the digestive tract, when air is swallowed, or when bacteria
in the large intestine breaks down undigested food.41 One study
demonstrated that most healthy adults excrete gas an average of 10 times a
day; however, other experts state that individuals may pass gas as much as 14
times each day.42,43
Although gas can affect anyone, it can be
problematic in pregnant women. One cause is progesterone, which is produced
during the early stages of pregnancy. Since progesterone relaxes the smooth
muscles, including the intestinal tract, digestion is slowed, leading to
increased stomach and intestinal gas. Slowed digestion also gives the
intestinal bacteria more time to ferment the undigested food, resulting in
more gas production.44

Pharmacologic Treatment
Gas in pregnant
women can be treated with nonprescription medications. Some products that
contain simethicone, a common treatment for gas, include Gas-X, Phazyme,
Mylanta Gas Softgels, and Mylicon drops. This OTC medication relieves gas in
the stomach and intestine by reducing the surface tension of gas bubbles. By
altering the surface tension, the gas bubbles break and are able to be
eliminated by belching or flatulence. Since simethicone is not absorbed from
the GI tract, there are no known systemic side effects. It is pregnancy
category C, and no congenital defects have been reported.37
Another treatment is
alpha-galactosidase. Products that contain this enzyme include Beano and Gaz
Away. This OTC medication hydrolyzes high-fiber foods and other foods that
contain oligosaccharides before they can be metabolized by the bacteria in the
large intestine. The recommended dose is adding about five drops to each
serving of problematic food or taking three tablets with each meal. Five drops
or three tablets contain 150 units of alpha-galactosidase. Drops should be
added to the food after it has cooled. Other OTC options are lactase
replacement products, such as Dairy Ease, Lactaid, and SureLac tablets.
Lactase replacement may be effective for patients who are intolerant to
lactose. These products break down lactose into glucose and galactose and are
to be taken when dairy products are ingested. As lactase replacement acts
locally in the gut, there are no reported systemic side effects. Pregnant
women should check with their physician before taking these OTC medications.
29,45
Many pregnant women can
control gas by making dietary changes. Table 8 lists nonpharmacologic
approaches to managing gas in pregnancy.29,46 One main change is to
limit the amount of gas-producing foods in the diet or totally eliminate foods
that are not tolerated.
Conclusion
Pregnant patients
may present with a variety of GI complaints. Nonpharmacologic and lifestyle
modifications can be recommended with or without concomitant pharmacologic
treatment. However, prior to recommending pharmacologic therapy, the patient
should be evaluated by a physician. Pharmacists represent a key part of the
health care team and can assess whether a pregnant patient's symptoms require
immediate physician referral. Additionally, pharmacists can assist
patients and physicians with product selection based on available safety data.
Once a product is selected, the pharmacist can counsel the patient on
appropriate dosage and usage.
References
1. Jewell D, Young G. Interventions for nausea and vomiting in early
pregnancy. Cochrane Database Sys Rev 2003;4:CD000145.
2. Hayakawa S, et al. Frequent presence of Helicobacter pylori genome in the
saliva of patients with hyperemesis gravidarum. Am J Perinatol.
2000;17:243-247.
3. American College of Obstetrics and Gynecology (ACOG). ACOG Practice
Bulletin Number 52: Nausea and vomiting of pregnancy. Obstet Gynecol.
2004;103:803-814.
4. Vutyavanich T, et al. Ginger for nausea and vomiting in pregnancy: a
randomized, double-masked placebo-controlled trial. Obstet Gynecol.
2001;97:577-582.
5. Keating A, Chez RA. Ginger syrup as an antiemetic in early pregnancy.
Altern Ther Health Med. 2002;8:89-91.
6. Backon J. Ginger in preventing nausea and vomiting of pregnancy; a caveat
due to its thromboxane synthetase activity and effect on testosterone binding.
Eur J Obstet Gynecol Reprod Biol. 1991;42:163-164.
7. Mazzotta P, Magee LA. A risk-benefit assessment of pharmacological and
nonpharmacological treatments for nausea and vomiting of pregnancy. Drugs
. 2000;59:781-800.
8. Richter JE. Review article: the management of heartburn in pregnancy.
Aliment Pharmacol Ther. 2005;22:749-757.
9. Larson JD, Patatanian E, et al. Double-blind, placebo-controlled study of
ranitidine for gastroesophageal reflux symptoms during pregnancy. Obstet
Gynecol. 1997;90:83-87.
10. Knudsen A, Lebech M, Hansen M. Upper gastrointestinal symptoms in the
third trimester of the normal pregnancy. Eur J Obstet Reprod Biol.
1995;60:29-33.
11. Fisher RS, Roberts GS, et al. Altered lower esophageal sphincter function
during early pregnancy. Gastroenterology. 1978;74:1233-1237.
12. Tytgat GN, Heading RC, et al. Contemporary understanding and management of
reflux and constipation in the general population and pregnancy: a consensus
meeting. Aliment Pharmacol Ther. 2003;18:291-301.
13. Marrero JM, Goggin PM, et al. Determinants of pregnancy heartburn. Br J
Obstet Gynaecol. 1992;99:731-734.
14. Ching C, Lam S. Antacids: indications and limitation. Drugs. 1994;
47: 305-317.
15. Witter FR, King TM, Blake DA. The effects of chronic gastrointestinal
medication on the fetus and neonate. Obstet Gynecol. 1981;58:79S-84S.
16. Smallwood RA, et al. Safety of acid-suppressing drugs. Dig Dis Sci.
1995;40(Suppl):63S-80S.
17. Weberg R, Berstad A, Ladehaug B. Are aluminum containing antacids during
pregnancy safe? Acta Pharmacol Toxicol. 1986;59(Suppl 7):63-65.
18. Mandel KG, Daggy BP, et al. Review article: alginate-raft formulations in
the treatment of heartburn and acid reflux. Aliment Pharmacol Ther.
2000;14:669-690.
19. Lindow SW, Regnell P, et al. An open-label, multicentre study to assess
the safety and efficacy of a novel reflux suppressant (Gaviscon Advance) in
the treatment of heartburn during pregnancy. Int J Clin Pract.
2003;57:175-179.
20. Magee LA, Inocencion G, et al. Safety of first trimester exposure to
histamine H2 blockers. A prospective cohort study. Dig Dis Sci.
1996;41:1145-1149.
21. Ruigomez A, Rodriguez LAG, et al. Use of cimetidine, omeprazole, and
ranitidine in pregnant women and pregnancy outcomes. Am J Epidemiol.
1999;150:476-481.
22. Parker S, Schade RR, et al. Prenatal and neonatal exposure of male rat
pups to cimetidine but not ranitidine adversely affects subsequent adult
sexual functioning. Gastroenterology. 1984;86:675-680.
23. Nielson GL, Sorensen HT, et al. The safety of proton pump inhibitors in
pregnancy. Aliment Pharmacol Ther. 1999;13:1085-1089.
24. Lalkin A, Loebstein R, et al. The safety of omeprazole during pregnancy: a
multicenter prospective controlled study. Am J Obstet Gynecol.
1998;179:727-730.
25. Kallen B. Delivery outcome after the use of acid-suppressing drugs in
early pregnancy with special reference to omeprazole. Br J Obstet Gynaecol
. 1998;105:877-881.
26. Nikfar S, Abdollahi M, et al. Use of proton pump inhibitors during
pregnancy and rates of major malformations. A meta-analysis. Dig Dis Sci
. 2002;47:1526-1529.
27. Diavcitrin O, Arnon J, et al. The safety of proton pump inhibitors in
pregnancy: a multicenter prospective controlled study. Aliment Pharmacol Ther.
2005;21:269-275.
28. DiPiro J, et al. Pharmacotherapy: A Pathophysiologic Approach. 5th
ed. New York, NY: McGraw-Hill; 2002:655-669.
29. Berardi R, McDermott J, et al. Handbook of Nonprescription Drugs.
14th ed. Washington, DC: American Pharmacists Association; 2004:335-359.
30. Forrester A. Diarrhea. Patient Self-Care (PSC). 2002:238-251.
31. Black R, Hill DA. Over-the-counter medications in pregnancy. Am Family
Phys. 2003;67:2517-2524.
32. Einarson A, Mastroiacovo P, et al. Prospective controlled multicenter
study of loper amide in pregnancy. Can J Gastroenterol.
2000;14:185-187.
33. Wald A. Constipation, diarrhea, and symptomatic hemorrhoids during
pregnancy. Gastroenterol Clin N Am. 2003;32:309-322.
34. Friedman JM, Polifka JE. Teratogenic Effects of Drugs: A Resource for
Clinicians. 2nd ed. Baltimore: The Johns Hopkins University Press; 2000.
35. Jewell D, Young G. Interventions for treating constipation in pregnancy.
Cochrane Database Syst Rev. 2001;2:CD001142.
36. Muller-Lissner SA, Kamm MA, et al. Myths and misconceptions about chronic
constipation. Am J Gastroenterol. 2005;100:232-242.
37. Briggs GG, et al. Drugs in Pregnancy and Lactation. 7th ed.
Philadelphia: Williams & Wilkins; 2005.
38. Baron TH, Ramirez B, Richter JE. Gastrointestinal motility disorders
during pregnancy. Ann Intern Med. 1993;118:366-375.
39. Thomson Micromedex. USPDI Drug Information for the Health Care
Professional: Volume 1. 25th ed. Taunton, MA: Quebecor World; 2005.
40. Quijano CE, Abalos E. Conservative management of symptomatic and/or
complicated haemorrhoids in pregnancy and the puerperium. Cochrane Database
Sys Rev. 2005;3:CD004077.
41. Tierney LM, et al. Current Medical Diagnosis and Treatment 2006.
Gastroenterology. University of Cincinatti library Web site. Available
at:www.accessmedicine.com/content.aspx?aID=6395. Accessed August 28, 2006.
42. Furne JK, Levitt MD. Factors influencing frequency of flatus emission by
healthy subjects. Dig Dis Sci. 1996;41:1631-1635.
43. Basotti G, et al. Flatus–related colorectal and anal motor events.
Dig Dis Sci. 1996;41:335-338.
44. Gas and bloating during pregnancy. Baby Center. February 2005. Available
at: www.babycenter.com/refcap/pregnancy/prenatal health/247.html. Accessed
February 16, 2006.
45. Clinical Pharmacology (online version). Accessed at University of
Cincinatti library Web site. Available at:www.cpip.gsm.com. Accessed August
28, 2006.
46. Health A to Z. Aetna InteliHealth Inc. February 2004. Available at:
www.intelihealth.com/IH/ihtIH/WSIHW000/8270/22025/346741.html. Accessed
February 16, 2006.
To comment on this article,
contact editor@uspharmacist.com.






