US Pharm. 2016;41(9)18-20.
Projected increases in life expectancy are the underlying basis for the expectation that over the next 50 years, the number of cancer patients will double from the present 1.3 million to 2.6 million.1,2 The majority of these patients will be >75 years of age.2 With regard to ovarian cancer, which affects mostly those individuals in perimenopause and postmenopause, approximately 1.3% of women in the general population will develop the condition.3,4 Incidence of ovarian carcinoma increases as age advances, with a peak occurring during the seventh decade of life; incidence remains elevated until the age of 80.5 Older women are more likely to present with advanced disease and are less likely to be offered radical surgery and chemotherapy.6 One population-based study indicated that women >75 years were significantly less likely than younger women to be given chemotherapy, receive care by oncology specialists, or undergo adequate primary cytoreductive surgery.7
Gene Mutations
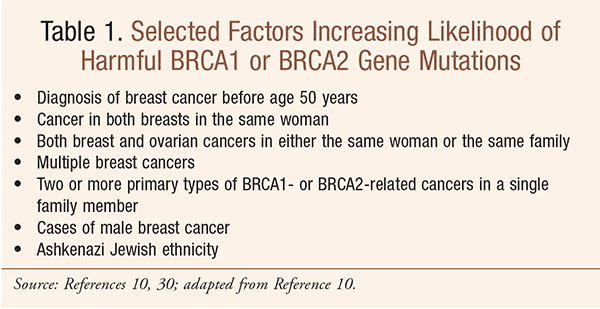
With regard to gene mutations, according to recent estimates, 39% of women who inherit the harmful BRCA1 mutation and 11% to 17% of women who inherit a harmful BRCA2 mutation will develop ovarian cancer by the age of 70.8,9 While screening asymptomatic women using ultrasonography and measurement of tumor markers (e.g., cancer antigen [CA] 125) can detect some cases of ovarian cancer, it has not been shown to improve outcome, even for high-risk subgroups, including women with BRCA mutations.3 However, women should be screened for abnormalities in the BRCA gene.3 In December 2013, the U.S. Preventive Services Task Force recommended that women who have family members with breast, ovarian, fallopian tube, or peritoneal cancer be evaluated to see if they have a family history that is associated with an increased risk of a harmful mutation in one of these genes (TABLE 1).10 One recent randomized, controlled trial demonstrated the value of implementing strategies that target women at high risk for ovarian cancer to ensure they are offered access to recommended care.11
Risk Factors and Clinical Manifestations
Ovarian cancers are histologically diverse, with 90% of ovarian malignancies in older women noted as epithelial adenocarcinomas.3,6 Risk factors that potentially increase the likelihood of ovarian cancer are outlined in TABLE 2, and include age >50 years, the use of fertility agents, and the use of hormone replacement therapy. Symptoms (TABLE 3) are usually absent in early stages and nonspecific in advanced stages.3 Due to the nonspecific nature of symptoms, vague complaints such as abdominal discomfort and bloating may be disregarded, attributed to gastrointestinal issues, and go unaddressed over time by patients and clinicians alike until detected at an advanced stage.


Diagnosis and Treatment
Evaluation usually includes hematologic and biochemical profiles, chest x-ray, ultrasonography (for suspected early cancers) or CT or MRI (for suspected advanced cancers), and measurement of CA125.3,6 Diagnosis is by histologic analysis, while staging is surgical.3 The standard of care for ovarian cancer includes initial surgical cytoreduction, such as debulking of the tumor, bilateral salpingo-oophorectomy, total hysterectomy, or infracolic omentectomy followed by platinum (e.g., cisplatin, carboplatin) and taxane (e.g., paclitaxel) chemotherapy.3
Prospective randomized trials compared cisplatin plus paclitaxel versus cisplatin plus cyclophosphamide, demonstrating the superiority of the paclitaxel-containing regimen.12,13 Subsequent prospective randomized trials compared paclitaxel/carboplatin versus paclitaxel/cisplatin, demonstrating decreased toxicity with the carboplatin regimen and no difference in efficacy.14,15 Due to these results, paclitaxel plus carboplatin is now considered first-line treatment for most patients with advanced ovarian cancer.5 Ascites as a recurrent condition/complication is a major problem requiring repeated paracentesis; to reduce fluid and limit recurrence, spironolactone therapy may be introduced.6
While it is not clear at present if age is a negative prognostic factor, in the elderly, pharmacodynamic changes can potentially decrease efficacy and increase toxicity of cytotoxic treatments.5 Of note, several studies have demonstrated that despite an age-related decrease in patients’ creatinine clearance of many drugs, total clearance does not change; this may be attributed to an increase in hepatic clearance.5,16-18
Although the majority of elderly patients are able to tolerate the standard of care for ovarian cancer, it is important to note that because functional status does not demonstrate a reliable correlation with either tumor stage or comorbidity, each patient’s comorbidities should be assessed independently.5 Ultimately, in elderly individuals with considerable comorbidity, tailoring the extent of surgery and the aggressiveness of chemotherapy to a patient’s disease acuity, symptoms, overall health, and life goals is imperative.5
Research Supporting Previous Reports: Treatment in the Elderly
One large study of chemotherapy tolerance in elderly oncology patients (median age of the 148 patients was 73 years [range: 70 to 84 years], 37% were >75 years) evaluated women with gynecologic malignancies treated between 1990 and 2000 in four centers, with almost 70% of participants treated for ovarian cancer.19 Of note, one or more comorbid conditions were present in nearly 80% of patients; of the patients who received first-line chemotherapy, 96 (64.9%) received a platinum-based therapy with no taxane, 42 (28.4%) received combined platinum/paclitaxel therapy, and 10 (6.8%) received a taxane-based regimen with no platinum.19 Researchers indicated that no significant differences were observed in performance status before, during, and at the time of completion of first-line chemotherapy; of the ovarian cancer patients, 74 (71.9%) received combination chemotherapy, and 38 (36.9%) received first-line treatment with a platinum agent and taxanes. It was reported that grade 3/4 hematologic toxicities were reported in 38.2% of patients, and 6.8% of them discontinued treatment due to toxicity. They concluded that no significant association was seen between the number of comorbidities and toxicities; furthermore, the study confirmed previous reports, which indicated that elderly patients with adequate renal and hepatic function tolerate standard chemotherapy regimens with equivalent toxicity profiles and no significant difference in treatment delays or discontinuations.20-23
Additional Commentary Regarding the Elderly
One commentary regarding the above summary noted that the majority of studies examining age in relation to clinical care and outcome for ovarian cancer broadly separate elderly from young patient cohorts based on a conventional demarcation of 65 years or older; however, there is considerable heterogeneity among patients within this group with regard to functional status and disease comorbidities.1 It is suggested that age should never serve as a substitute for the appropriate application of functional status.1 Bryan and Berek note that the myth of chronologic age as the sole determinant of function maintains a strong hold in both patients and physicians despite evidence to the contrary; he adds, “ageism serves as the barrier between what is known to be clinically true, and what is actually practiced.”1
Additional commentary notes that data suggest older individuals may be denied the full extent of modern treatment for ovarian cancer, since age is widely thought to be associated with a poorer outcome and an unacceptable rate of treatment-related complications.24 However, age itself should not be a barrier to appropriate treatment or to inclusion into clinical trials, and treatment plans should be based on the physiologic rather than chronologic age of the patient.24 It is suggested that well-founded recommendations need to be complemented by two additional considerations:24
Biologic interaction: It should not be dismissed that age by itself, through poorly defined mechanisms, may contribute to a more aggressive or less responsive disease.
Practical assessment: Simple questionnaires can be used, such as one recommended by the National Comprehensive Cancer Network guidelines, the Vulnerable Elders Survey (VES-13), and evaluations of physical function such as the Get Up and Go test.25,26
These recommendations encompass the consideration that functional status, assessed as the ability to perform activities of daily living and instrumental activities of daily living, is an independent prognostic factor for the risk of chemotherapy-induced myelotoxicity.24,26 Further, the author suggests that a simple estimate of life expectancy may be obtained with the comprehensive geriatric assessment.24,27 Some laboratory tests, including serum concentrations of interleukin-6 and D-dimer, may predict the risk of functional dependence and mortality.24,28
Patient Education
One interesting approach to patient education in gynecologic oncology involved the creation of a multidisciplinary team consisting of physicians, pharmacists, advanced-practice providers, nurses, administrators, health-education specialists, and members of the quality improvement department to establish a Shared Medical Appointment and Readiness Teaching (SMART) program for all patients beginning chemotherapy with platinum- and/or taxane-based regimens.29 A standardized chemotherapy-education presentation was developed and patients were provided with a tool kit that consisted of chemotherapy-medication education, a guide to managing adverse effects, advance directives, and contact information for the medical center.29 The researchers noted that this particular model of care provides patient education as an integral part of a matrix of social support that empowers patients. They indicated that shared medical appointments for oncology patients initiating chemotherapy are not only feasible, but well-accepted.29
Conclusion
Although pharmacodynamic changes may decrease efficacy and increase toxicity of cytotoxic treatments, standard-of-care chemotherapy for older women with ovarian cancer may be feasible and well-tolerated. Treatment plans should be based on the physiologic rather than the chronologic age of the patient; it is recommended that patients should be assessed based on functional status rather than age alone, with each patient’s comorbidities assessed independently. In an older adult with considerable comorbidity, tailoring the extent of surgery and the aggressiveness of chemotherapy to the person’s disease acuity, symptoms, overall health, and life goals is suggested. Individualized patient care may help reduce the incidence of adverse drug events in systemic cancer therapy, supporting the optimism about the expanded role of the pharmacist on multidisciplinary oncology teams. Experts recommend a collaborative effort to enter older adults into treatment protocols and encourage cooperation between geriatricians and oncologists for pretreatment assessment and improvement in treatment guidelines for the geriatric population.
REFERENCES
1. Bryan DN, Berek JS. Commentary. Ovarian cancer, Oncology. August 1, 2003. www.cancernetwork.com/ovarian-cancer/commentary-bryanberek-ovarian-cancer-elderly-women. Accessed July 25, 2016.
2. Edwards B, Howe H, Ries L, et al. Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on US cancer burden. Cancer. 2002;94:2766-2792.
3. Ramirez PT, Gershenson DM. Ovarian cancer. Merckmanuals.com. Last full review/revision May 2013. www.merckmanuals.com/professional/gynecology-and-obstetrics/gynecologic-tumors/ovarian-cancer. Accessed August 17, 2016.
4. Howlader N, Noone AM, Krapcho M, et al. (eds). SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site April 2014. Updated December 2014.
5. Lambrou NC, Bristow RE. Ovarian cancer in elderly women. Oncology. August 1, 2003. www.cancernetwork.com/ovarian-cancer/ovarian-cancer-elderly-women. Accessed July 25, 2016.
6. Cooper TK, Smith OM. Gynecologic disorders in the elderly. In: Fillit HM, Rockwood K, Woodhouse K, eds. Brocklehurst’s Textbook of Geriatric Medicine and Gerontology. 7th ed. Philadelphia, PA: Saunders Elsevier; 2010:716-725.
7. Cress R, O’Malley C, Leiserowitz G, et al. Patterns of chemotherapy use for women with ovarian cancer: a population-based study. J Clin Oncol. 2003;21:1530-1535.
8. Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117-1130.
9. Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329-1333.
10. U.S. Preventive Services Task Force. BRCA-related cancer: risk assessment, genetic counseling, and genetic testing. AHRQ Publication No. 12-05164-EF-3. December 2013. Update July 2015. www.uspreventiveservicestaskforce.org/uspstf12/brcatest/brcatestsumm.htm. Accessed August 14, 2016.
11. Drescher CW, Beatty JD, Resta R, et al. The effect of referral for genetic counseling on genetic testing and surgical prevention in women at high risk for ovarian cancer: results from a randomized controlled trial. Cancer. July 22, 2016. [Epub ahead of print].
12. McGuire WP, Hoskins WJ, Brady MF, et al: Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1-6.
13. Stuart G, Bertelson K, Mangioni C, et al. Updated analysis shows a highly significant improved overall survival (OS) for cisplatin-paclitaxel as first line treatment of advanced ovarian cancer; mature results of the EORTC-GCCG, NOCOVA, NCTC, CT and Scottish Intergroup Trial (abstract). Proc Am Soc Clin Oncol. 1998;17:361.
14. du Bois A, Lueck HJ, Meier W, et al. Cisplatin/paclitaxel vs carboplatin/paclitaxel in ovarian cancer: update of an Arbeitsgermeinschaft Gynaekologische Onkologie (AGO) Study Group Trial (abstract 1374). Proc Am Soc Clin Oncol. 1999;18:356a, 1999.
15. Ozols RF, Bundy BN, Fowler J, et al: randomized phase II trial of cisplatin/paclitaxel versus carboplatin/paclitaxel in optimal stage III epithelial ovarian cancer: a Gynecologic Oncology Group trial (GOG 158) (abstract 1373). Proc Am Soc Clin Oncol. 1999;18:356a.
16. Ceccaroni M, De Iaco P, Scambia G, et al. Chemotherapy in elderly gynecologic cancer patients aged over 70 years: facts and controversies. Womens Oncol Rev. 2002;2:385-394.
17. Balducci L, Extermann M. Cancer chemotherapy in the older patient. Cancer. 1997;80:1317-1322.
18. Borkowski JM, Duerr M, Donehower RC, et al. Relation between age and clearance rate of nine investigational anticancer drugs from phase I pharmacokinetic data. Cancer Chemother Pharmacol. 1994;33:493-496.
19. Ceccaroni M, D’Agostino G, Ferrandina G, et al. Gynecologic malignancies in elderly patients: Is age 70 a limit to standard-dose chemotherapy? An Italian retrospective toxicity multicentric study. Gynecol Oncol. 2002;85:445-450.
20. Edmonson JH, Su J, Krook JE: Treatment of ovarian cancer in elderly women. Mayo Clinic North Central Cancer Treatment Group studies. Cancer. 1993;71(2 suppl):615-617.
21. Gronlund B, Hogdall C, Hansen HH, et al. Performance status rather than age is the key prognostic factor in second-line treatment of elderly patients with epithelial ovarian carcinoma. Cancer. 2002;94:1961-1967.
22. Gershenson DM, Follen Mitchell M, Atkinson N, et al. Age contrasts in patients with advanced epithelial ovarian cancer: the M. D. Anderson Cancer Center experience. Cancer. 1993;71:638-643.
23. Bicher A, Sarosy G, Kohn E, et al. Age does not influence taxol dose intensity in recurrent carcinoma of the ovary. Cancer. 1993;71:594-600.
24. Balducci L. Commentary. Ovarian cancer in elderly women. August 1, 2003. www.cancernetwork.com/ovarian-cancer/commentary-balducci-ovarian-cancer-elderly-women. Accessed July 25, 2016.
25. Balducci L, Yates J. General guidelines for the management of older patients with cancer. Oncology. 2002;14:221-227.
26. Repetto L, Balducci L. A case for geriatric oncology. Lancet Oncol. 2002;3:289-297.
27. Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285:2987-2994.
28. Cohen HJ, Harris T, Pieper CF. Coagulation and activation of the inflammatory pathway in the development of functional decline and mortality in the elderly. Am J Med. 2003;114:180-189.
29. Prescott LS, Dickens AS, Guerra SL, et al. Fighting cancer together: development and implementation of shared medical appointments to standardize and improve chemotherapy education. Gynecol Oncol. 2016;140(1):114-119.
30. National Institutes of Health. National Cancer Institute. BRCA1 and BRCA2: Cancer risk and genetic testing. Reviewed April 1, 2015. www.cancer.gov/about-cancer/causes-prevention/genetics/brca-fact-sheet. Accessed August 14, 2016.
31. Ovarian cancer. Clinical pharmacology. 2016 Elsevier/Gold Standard. http://clinicalpharmacology-ip.com/forms/patiented/drughandouts.aspx?di=11855&l=1&p=d. Accessed August 14, 2016.
To comment on this article, contact rdavidson@uspharmacist.com.