US Pharm. 2009;34(12):32-41.
Hepatitis is an inflammation of the liver that is often caused by one of five hepatitis viruses: A, B, C, D, or E. Hepatitis A and E are commonly contracted by ingestion of contaminated food or water, while transmission of hepatitis B and C occurs by parenteral contact with infected body fluids. No specific drug therapy is available for the treatment of hepatitis A or E. Hepatitis B and C infections are worldwide concerns because they often lead to chronic liver disease and even death. Much effort has been dedicated to global eradication of these diseases through education and vaccination. Still, viral hepatitis is a major public health concern. While both hepatitis B and C are associated with significant morbidity and mortality, the therapeutic approach to treatment varies by virus type, and each will be discussed separately.
Hepatitis B
In the United States, only 4,519 new acute hepatitis B (HBV) cases were reported to the Centers for Disease Control in 2007.1 This represents a significant decrease from the early 1980s, when the number of reported cases of HBV each year was generally greater than 25,000. A national immunization strategy instituted in the early 1990s was largely responsible for the sharp decline in new HBV cases. The incidence of HBV in the U.S. is now 1.5 cases per 100,000 persons, which is an all-time low.1 Still, HBV remains an important worldwide public health concern, with an estimated 1 million deaths each year due to HBV sequelae.
HBV is a DNA virus transmitted by percutaneous or mucosal contact with infectious blood or body fluids. Accordingly, intravenous drug users and those who practice unprotected sex are at increased risk of HBV infection. Infants born to infected mothers are also at greater risk of becoming infected with HBV. Morbidity and mortality associated with chronic HBV infection are connected to the development of cirrhosis or hepatocellular carcinoma (HCC). Worldwide, approximately 75% of HCC cases are due to chronic HBV infection.2
Clinical Characteristics and Diagnosis: HBV infection is often characterized by acute hepatitis, with approximately 5% of these infections progressing to chronic disease. The presence of serum hepatitis B surface antigen (HBsAg) indicates infection with HBV, and antibodies against HBsAg indicate recovery. Hepatitis B e antigen (HBeAg) is elevated during periods of active viral replication, but may be absent in chronic hepatitis due to a DNA mutation of the virus responsible for hepatitis B. In fact, HBeAg-negative chronic HBV is generally linked to more serious liver disease and a limited sustained response to antiviral therapy. The presence of anti-HBc (core antibody) indicates HBV infection at some point in time as opposed to positivity for HBV surface antibody, which may indicate humoral immunity in response to HBV vaccination.3 Signs and symptoms of infection depend on the age of the patient, and many patients may be asymptomatic initially. Symptoms may be nonspecific and do not aid in differentiating HBV from other forms of acute hepatitis. Patients may complain of fever, fatigue, and abdominal pain. Other findings may include anorexia, nausea, vomiting, dark urine, clay-colored stools, joint pain, and jaundice.
Management: The goals of treating chronic HBV are to achieve sustained suppression of viral replication and prevent progression of liver disease.4 Response to treatment can be measured using biochemical (normalization of alanine aminotransferase [ALT]), virologic (clearance of HBV DNA), serologic (loss of HBeAg, HBeAg seroconversion), or histologic markers. All available treatment options have advantages and disadvantages, and drug therapy selection should take into account cost, efficacy, safety, risk of drug resistance, and method of administration.
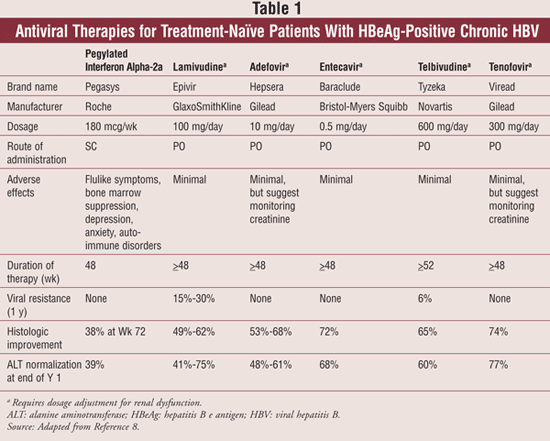
Antiviral Treatment: For the minority of patients who develop chronic HBV infection, there are currently eight drugs approved by the FDA for the treatment of chronic hepatitis B: conventional interferon (IFN) alpha-2b, lamivudine, adefovir dipivoxil, pegylated interferon (pegIFN) alpha-2a, entecavir, telbivudine, and tenofovir disoproxil fumarate. The prototype antiviral used for the treatment of HBV is IFN. IFN is effective at inducing a virologic, histologic, and biochemical response at a dose of 10 million international units (IU) three times weekly or 5 million IU daily. Adverse reactions classified as severe were reported in 21% to 44% of patients, the most common being fever, fatigue, bone marrow suppression, and alopecia.5 As a result, pegIFN has largely replaced IFN alpha-2b due to its longer half-life and improved tolerability. IFN alpha-2b is administered as a once-weekly subcutaneous injection for a duration of 48 weeks.
While treatment with IFN yields a highly durable viral response, nucleoside/nucleotide analogues have become the primary treatment modality for chronic HBV due to their ease of use and tolerability. Lamivudine (LAM) is a convenient oral therapy for treatment of chronic HBV with modest efficacy and minimal side effects. Unfortunately, LAM has been associated with a high rate of resistance (up to 70% after 5 years) and virologic breakthrough, thus limiting its usefulness as a first-line agent for chronic HBV.6 Conversely, LAM is well tolerated and may be used in select circumstances such as HIV coinfection. For patients in whom LAM resistance is suspected or who have received therapy for more than 2 years, alternative therapies such as entecavir (ETV) and tenofovir disoproxil fumarate (TDF) have proven effective.7
Adefovir dipivoxil (ADV) is another well-tolerated oral therapy for treatment of chronic HBV with slightly less viral resistance than LAM. Primary nonresponse to ADV occurs at a rate of 20% to 50%, presumably due to the low dosage used in chronic HBV treatment.8 ADV may cause nephrotoxicity at higher doses, thus limiting its utility as a first-line agent. It should be avoided in combination with TDF due to the additive risk of nephrotoxicity. Monitoring of serum creatinine every 3 months is recommended for patients with medical conditions that may lead to renal dysfunction and for patients receiving treatment with ADV for more than 1 year.6
ETV is a nucleoside analogue with excellent potency that is associated with minimal viral resistance in untreated patients. ETV has been used successfully in patients with LAM-resistant disease, though efficacy is reduced when compared to treatment of nucleoside analogue-naïve patients for whom a higher dosing strategy is used.9 Like other nucleoside/nucleotide analogues, ETV must be dose adjusted in renal dysfunction.
Telbivudine (LdT) is more potent than LAM at HBV suppression, but it has a high level of cross-resistance with LAM. Additionally, resistance to LdT develops rapidly, especially with treatment duration beyond 1 year.6 Though this drug is generally well tolerated, myopathy and peripheral neuropathy have also been associated with its use. These safety and viral resistance concerns have caused LdT to have an uncertain role in contemporary management of HBV.
The most recently approved therapy for treatment of chronic HBV is TDF. When compared to ADV, treatment with TDF yielded a significantly greater histologic response and normalization of ALT.7 TDF is useful for treatment of LAM-resistant HBV. Renal dysfunction and decreases in bone mineral density have been reported infrequently.7
Hepatitis C
Viral hepatitis C (HCV) affects approximately 4 million persons in the U.S. alone, with a worldwide disease burden estimated at 180 million.10 Of those infected in the U.S., the vast majority have active disease. While some individuals may clear the disease, 75% develop chronic infection. Infection with HCV is often insidious, and affected individuals are often asymptomatic during much of the course of the disease. Significant morbidity is related to the development of cirrhosis, which currently affects over 600,000 individuals. HCV-related mortality is attributed to complications of end-stage liver disease as well as death from development of HCC. The rate of newly diagnosed HCV infection has fortunately declined to about 17,000 cases annually, mainly due to improved screening for HCV in blood products, which began around 1992. Additionally, heightened awareness of the risks of needle-sharing among injection drug users has reduced the transmission of HCV; still, acquisition of new HCV continues to occur primarily in injection drug abusers.11 Despite the decline in new cases, HCV-associated mortality continues to rise due to clinical progression of HCV.
Clinical Characteristics and Diagnosis: Acute HCV infection usually goes undetected, as symptoms are nonspecific in nature and may include anorexia, abdominal discomfort, and nausea and vomiting, along with elevated hepatic transaminases and, less commonly, jaundice.11,12 For this reason, diagnosis cannot be made based on risk factors and clinical presentation. Specific serologic testing for the presence of anti-HCV antibodies is indicated in any at-risk individual (TABLE 1) and establishes exposure to the disease.13 Virologic testing for the presence of HCV-ribonucleic acid (HCV-RNA) along with viral genotyping is also indicated in all patients who test positive for HCV, particularly in those patients being considered for antiviral therapy.13
Management of Patients With HCV: Determining a patient’s specific HCV genotype is mandatory because it aids in predicting the likelihood of response to drug therapy and determines optimal treatment duration as well.12,13 There are a total of six distinct, numbered HCV genotypes. Genotype 1 accounts for about 70% of the disease in the U.S., followed by genotypes 2 and 3. Genotype 1 is generally more refractory to antiviral drug therapy, and comparative studies have demonstrated substantially lower treatment response rates when compared to rates among those infected with genotypes 2 or 3.11,13 Genotyping is therefore valuable in evaluating the risk-to-benefit ratio of antiviral therapy. Because anti-HCV medications put patients at risk for significant treatment toxicities, a thorough assessment of a patient’s overall medical and mental health is necessary to determine if contraindications to treatment are present. Contraindications to combination HCV treatment include decompensated liver disease; pregnancy; uncontrolled depression or neuropsychiatric illness; history of autoimmune hepatitis; severe uncontrolled comorbid illnesses such as seizure disorders, thyroid disorders, diabetes, and active coronary disease; age less than 2 years; anemia; post solid-organ transplantation; or unwillingness to use adequate contraception.13
In general, patients who are considered candidates for combined anti-HCV therapy include adult patients with chronic HCV infection (≥6 months) who have evidence of chronic hepatitis based on liver biopsy with compensated liver disease and acceptable laboratory values on serum chemistry. In addition, due to high rates of neuropsychiatric side effects of combination ribavirin-INF (i.e., depression, and anxiety in up to one-thid of patients), it is advisable to screen for any occult psychiatric illness prior to therapy. Patients must also be willing and able to adhere to treatment.
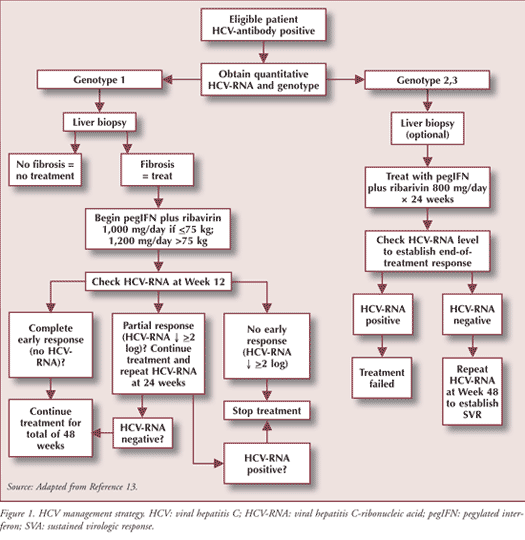
Antiviral Treatment: Subcutaneous pegIFN alpha combined with oral ribavirin is the mainstay of treatment of HCV virus infection with no clear evidence that one product is superior to the other (see FIGURE 1). While monotherapy with IFN may be used in individualized cases where ribavirin is contraindicated, ribavirin should never be used as monotherapy. Goals of treatment include early virologic response (EVR; undetectable HCV-RNA after 12 weeks of treatment), end-of-treatment response (ETR), and a sustained virologic response (SVR), defined as undetectable HCV-RNA 24 weeks after cessation of antiviral therapy.13 Additional goals are to minimize treatment-related toxicities, which may emerge on therapy while maintaining efficacy.
Both pegIFN products approved for use in the U.S., pegINF alpha-2b and pegINF alpha-2a, have demonstrated superior efficacy to standard interferon plus ribavirin.13 These products are similar in that both drugs have added various polyethylene glycol side chains to the parent molecule to increase the duration of pharmacologic activity. It is important to note that there are differences with respect to dosing, frequency, and how each product is combined with ribavirin. PegINF alpha-2b is given, in combination with ribavirin, as a weight-based dose of 1.5 mcg/kg, while a fixed dose of 180 mcg is used with pegINF alpha-2a. Both are given as weekly subcutaneous injections. Standard INF alphacon-1 is also approved for treatment in HCV in a fixed dosage given subcutaneously three times weekly alone or in combination with ribavirin. Adverse reactions to INF products are common and may lead to patient intolerance as well as reduced efficacy due to the need for dose reductions. Commonly experienced side effects attributable to INF include flulike symptoms, myelosuppression (anemia, neutropenia, and/or thrombocytopenia), psychiatric adverse effects, injection-site reactions, alopecia, anorexia, and sleep abnormalities.14 Toxicities generally associated with ribavirin include hemolytic anemia, fatigue, dermatologic reactions, and precipitation of gout. Ribavirin is also an abortifacient and is carcinogenic and teratogenic. As a result, adequate contraception must be present with female patients as well as female partners of male patients who are of childbearing age and should be continued for 6 months following treatment cessation.15
Monitoring and Follow-up: Duration of therapy and disease monitoring are dependent on HCV genotype, with genotype 1 generally requiring a longer course of therapy. In genotype 1–infected patients started on pegIFN/ribavirin, quantitative HCV-RNA is measured at baseline and at 12 weeks after treatment (see FIGURE 1). Patients with an EVR should continue treatment for a total of 48 weeks and then be assessed for an ETR. HCV-RNA testing is repeated 24 weeks following treatment cessation to determine presence of an SVR. If partial EVR is attained at Week 12 (HCV-RNA reduction of ≥2 log), treatment should be continued for an additional 12 weeks. A negative HCV-RNA test at 24 weeks means treatment should be continued for a total of 48 weeks. If no response is seen after 12 or 24 weeks, then treatment should be discontinued as the benefit is unlikely. In genotype 2 or 3 patients, HCV-RNA is not assessed until 24 weeks of therapy are complete.13 If HCV-RNA is not detected, then assessment for SVR is performed at 48 weeks. If HCV is still detectable, treatment failure has occurred. Efficacy defined by attainment of SVR is roughly 75% for genotypes 2 and 3 and 50% for genotype 1.13
Ongoing monitoring for both INF and ribavirin toxicity is indicated during treatment. Baseline CBC, serum creatinine, transaminases, thryoid-stimulating hormone (TSH), and pregnancy testing should be assessed at baseline. TSH should be monitored every 12 weeks on therapy, while other chemistries may be assessed monthly for the first 12 weeks, then every 2 months until the end of treatment unless specific toxicity issues warrant more frequent visits. Specific dosage reductions or treatment cessation for ribavirin is necessary with the development of anemia and or severe renal failure.15 Dosage reductions of pegIFN may also be necessary with elevated liver enzymes or renal insufficiency.
Patient Counseling
Pharmacists play an important role in the care of patients infected with viral hepatitis. The pharmacist may provide medication counseling and follow-up to ensure adherence or identify problems (e.g., drug toxicities) that emerge during therapy. All patients should be counseled to abstain from alcohol and avoid OTC drugs or supplements that may be hepatotoxic (e.g., high doses of acetaminophen). Counseling should include strategies to minimize disease prevention, such as use of barrier contraceptive methods or avoidance of high-risk behaviors. Immunization against HBV also plays a key role in primary prevention of HBV infection, and hepatitis B vaccination may be provided directly by pharmacists in many states.
REFERENCES
1. Centers for Disease Control and Prevention. Hepatitis B: FAQs for health professionals. www.cdc.gov/hepatitis/HBV/HBVfaq.htm#overview. Accessed October 2, 2009.
2. Marcellin P. Hepatitis B and hepatitis C in 2009. Liver Int. 2009;29(supp1):1-8.
3. Liaw YF. Natural history of chronic hepatitis B virus infection and long-term outcome under treatment. Liver Int. 2009;29(supp1):100-107.
4. Corey RL. Update on pharmacotherapy of chronic Hepatitis B and C. In: Richardson M, Chant C, Cheng JW, et al, eds. Pharmacotherapy Self-Assessment Program. 6th ed. Gastroenterology and Nutrition. Lenexa, KS: American College of Clinical Pharmacy; 2009:1-20.
5. Intron A [package insert]. Kenilworth, NJ: Schering-Plough; 2008.
6. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662.
7. Jenh AM, Thio CL, Pham PA. Tenofovir for the treatment of hepatitis B virus. Pharmacother. 2009;29(10):1212-1227.
8. Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500.
9. Liaw YF, Leung N, Kao JH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2(3):263-283.
10. Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705-714.
11. Centers for Disease Control and Prevention. Hepatitis C: FAQs for health professionals. www.cdc.gov/hepatitis/HCV/HCVfaq.htm#section1. Accessed September 7, 2009.
12. Kowdley KV. Hepatitis C. In: Floch MH, Floch NR, Kowdley KV, et al, eds. Netter’s Gastroenterology. Carlstadt, NJ: Icon Learning Systems; 2005.
13. Ghany MG, Strader DB, Thomas D, et al. AASLD practice guideline: diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1373.
14. Peg-intron [package insert]. Kenilworth, NJ: Schering-Plough; 2009.
15. Copegus [package insert]. Nutley, NJ: Roche Laboratories; 2009.
To comment on this article, contact rdavidson@jobson.com.





