US Pharm. 2016;41(9):38-41.
ABSTRACT: Pelvic inflammatory disease (PID) remains a relevant public health concern due to long-term effects on reproductive potential. Despite being the most common gynecologic infection, programs focusing on prevention are lacking. Given the correlation between PID and sexual activity, practitioners should be cognizant of this disease in adolescents and young adults. The treatment of PID should include antimicrobials with activity against common sexually transmitted pathogens as well as other vaginal microflora.
Pelvic inflammatory disease (PID) is a polymicrobial infection of the female genital tract that frequently results in acute or chronic pelvic pain, as well as infertility in women.1,2 The CDC estimates that nearly 1 million cases of PID are diagnosed each year, with 20% of cases occurring in adolescents, making it the most common gynecologic infection in the United States.1,3
Sexually active women aged 15 to 24 years comprise 25% of the sexually active population; however, they represent nearly 50% of the 18.9 million sexually transmitted diseases (STDs) reported in the U.S. each year. Younger women are at a greater risk for PID development than older women with greatest risk in those aged 15 to 20 years.4 From 2006-2010, approximately 5% of women reported being treated for PID in their lifetime,1 yet actual incidence may be higher due to many subclinical infections.
Risk Factors
Risk for PID is greatest in adolescence and young adulthood (age <25 y).5 Additionally, patients who engage in sexual activity at an early age (<15 years), have sex during menstruation, participate in casual sex while traveling, have a new sexual partner within the past 12 months, and/or multiple sexual partners are at increased risk.5-7 In rare cases, PID can occur in adolescent women who have not started menstruation or engaged in sexual activity.5 Use of contraception, vaginal douching, and a history of PID will also increase the patient’s risk for PID.5 PID also occurs more frequently in African-American women, but exact causes increasing infection rates are unknown.7
Intrauterine devices (IUDs) have been identified as a risk factor for PID; however, the newer generation levonorgestrel and copper IUDs have a decreased risk comparatively. Rates of PID have been found to be higher with the copper IUD in women <25 years of age.8 Risk peaks after the first month of insertion; women should be counseled about the small increase in risk. Women should be screened via history and physical examination for risk of PID prior to IUD insertion. Those with increased risk should be tested; however, IUD placement does not need to be delayed. As described below, PID can also be treated without removal of an IUD, unless the patient requests removal.8
Adolescent females are often considered poor candidates for IUDs as contraception due to the perceived risk of PID; however, both the World Health Organization (WHO) and the CDC view benefits likely outweighing real or theoretical risks.4 Thus, IUDs can be used in both nulliparous women and adoles-cents. IUDs should be offered to teenagers with a thorough sexual history, effective STD screening, and follow-up with age-specific counseling.4
Signs and Symptoms
Approximately 60% of patients with PID are asymptomatic.2 This predisposes patients to long-term sequelae if left untreated, including chronic pelvic pain and infertility in nearly 20% of patients. Unfortunately, symptoms are generally nonspecific and may range from mild abdominal pain to severe pelvic pain.1,5,9 A recent report found that rates of PID infection were higher in servicewomen ages 17 to 20 years who had a chlamydia diagnosis after basic training,10 which may represent a patient population where more efforts are needed for screening and education.
Cardinal symptoms include cervical motion tenderness, uterine tenderness, or adnexal tenderness.1,2,11 Other symptoms patients may experience include pelvic pain and tenderness, lower abdominal pain and tenderness, pain with sexual intercourse, irregular menstrual bleeding, or purulent vaginal discharge.1,2,11 Patients may also experience nonspecific symptoms such as lower back pain, nausea, and vomiting.9 When patients do report symptoms, 36% report mild-to-moderate symptoms and 4% report severe symptoms.11 Most women will present with lower abdominal or pelvic pain, although it is frequently mild, leading to a missed diagnosis or patients not seeking care.9
PID may also present with generalized systemic inflammation as a result of a suspected or known infection. Symptoms manifesting from these responses include an oral temperature >38.3°C (101°F), elevated erythrocyte sedimentation rate or C-reactive protein, or presence of white blood cells on microscopy of vaginal secretions.1,2,11
Pathophysiology and Diagnosis
Diagnosis of PID is made by clinical examination; there is no specific test confirming PID diagnosis.10 PID should always be part of the differential diagnosis in women aged 15 to 44 years who present with lower abdomen or pelvic pain.4,9 It is estimated that 10% to 20% of women with chlamydial or gonorrheal infections develop PID if untreated, which is likely to occur given that 80% to 90% of women with a chlamydial infection and 10% with a gonorrheal infection are asymptomatic.9 Despite a growing focus on antimicrobial stewardship, practitioners should initiate treatment when there is a reasonable suspicion of PID due to long-term sequelae and high risks of a missed diagnosis.4 Currently, the CDC recommends that providers screen all sexually active females younger than 25 years old, and older women with risk factors (e.g., new sexual partner, multiple sexual partners, or a sexual partner with an STD) for both chlamydia and gonorrhea.1 All women diagnosed with acute PID should be tested for HIV, gonorrhea, and chlamydia.1
In healthy women, the predominant vaginal-endocervical flora microbe is Lactobacillus. It is thought that Lactobacillus serves an important role in maintaining a healthy vaginal flora, acting as a defense mechanism by inhibiting colonization of pathogenic bacteria.9 Approximately 25% of asymptomatic women lack Lactobacillus-dominated flora, with nearly 50% having a vaginal pH >4.5.12 A dearth of Lactobacillus dominance may contribute to overgrowth of pathogenic bacteria, resulting in the subsequent development of PID.9 Bacterial vaginosis (BV) is a clinical syndrome where alternation in the vaginal flora occurs, with declining Lactobacilli. BV-associated organisms have been found to be associated with PID, and BV is an independent risk for both STDs and PID.13,14 It is unclear if identification and treatment of BV reduce the incidence of PID.1
PID is most commonly associated with Chlamydia trachomatis or Neisseria gonorrhoeae; however, causation by these pathogens is decreasing, with <50% of acute PID cases testing positive for either of these organisms.1,5 Other associated pathogens include Haemophilus influenzae, Gardnerella vaginalis, Group B Streptococcus, Mycoplasma genitalium, Ureaplasma urealyticum, and Cytomegalovirus (CMV), among many other potential pathogens.1,5 Patients with IUDs have been found to have greater infection rates with Peptostreptococcus and Fusobacterium species, which may be associated with complicated PID.9
PID is not limited to pathogens found in the vagino-cervical endogenous flora due to the ascending infection from the cervix and vagina to upper genital structures.5 Microorganisms implicated in PID are believed to spread to upper genital tract tissues to cause further infection in a multitude of ways. Infection may travel intra-abdominally from the cervix to the endometrium and then into the peritoneal cavity, may travel through lymphatic systems (e.g., infection of the parametrium from an IUD), or through hematogenous routes, but this last route is thought to be rare.9
Young adolescent females (<15 years of age) are at greatest risk for PID due to differences in cell composition in the genital tract. Less differentiated epithelial cells are less resistant to gonococcal and chlamydial infections when compared with cells seen in older adolescents and adult women. This is further complicated by immune system reactions to chlamydial infection, resulting in inflammation and subsequent chronic injury to genital tract tissue.5
Treatment
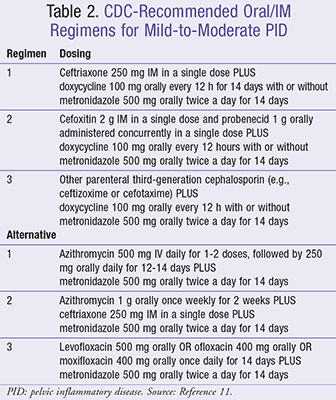
The treatment of PID can be divided into inpatient and outpatient treatment strategies as shown in TABLES 1 and 2, respectively.11 In females with mild-to-moderate disease, IV regimens appear to have equal efficacy to oral regimens.15 Patients who fail outpatient therapy are classified as complicated (e.g., tubo-ovarian abscess, pregnancy, unable to take oral medications) or are deemed to be surgical emergencies, should be considered for inpatient therapy. Patients who are treated with an IV regimen initially can often be transitioned to oral or IM therapy within 24 to 48 hours. Regardless of patient status, the selected regimen should provide adequate coverage of commonly implicated pathogens.15
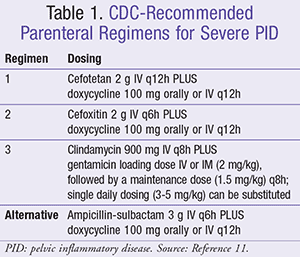
Several antimicrobial regimens have demonstrated efficacy in achieving clinical and microbiologic cure in randomized clinical trials.16,17 Notably, most regimens contain at least one agent with activity against anaerobic organisms due to some association with PID in both in vivo and in vitro studies. Some patients may also have concurrent BV, thus necessitating the addition of metronidazole. Because of this as well as the potential role of anaerobic pathogens in PID, the use of agents with anaerobic activity is recommended for the guidelines. N gonorrhoeae is also particularly problematic because of increasing rates of resistance to cephalosporins.18 Due to concerns for treatment failure, fluoroquinolone therapy should only be utilized when parenteral therapy is not feasible or patient allergies preclude other therapies from being selected. Doxycycline, a common component of both inpatient and outpatient regimens, should be administered orally whenever possible as it demonstrates similar bioavailability regardless of route and is caustic to veins when administered IV. Data using azithromycin in the treatment of PID is sparser and may not always be reliable against M genitalium and C trachomatis infections.19-21
Finally, adjunctive therapies such as use of nonsteroidal anti-inflammatory drugs (NSAIDs) or removal of IUDs does not improve clinical outcome.22,23 A systematic review comparing PID treatment courses in women who retained IUDs versus women with removal of IUDs overall showed comparable, if not better, outcomes in duration of hospitalization and improvement of clinical signs and symptoms.9 Hormonal contraceptives also offer benefit in prevention of PID, reducing the risk by 50% to 60%; subsequently, this reduces the risk of ectopic pregnancy and infertility issues associated with PID. When patients do become infected with PID during hormonal contraceptive use, PID appears to have less severe inflammation.24
As pharmacists, it is imperative to be cognizant of the potential adverse effects associated with these regimens. Doxycycline, a common component in many of the regimens, has the potential to cause photosensitivity as well as gastrointestinal (GI) upset. Patients should be counseled to wear sunscreen if they plan to be outdoors for extended periods of time and take with a meal to minimize GI symptoms. Metronidazole may cause a disulfiram reaction if combined with alcohol or alcohol-containing products and thus patients should be counseled on avoidance of alcohol during the treatment course. This drug can also be associated with taste disturbance and urine discoloration. Levofloxacin and other fluoro-quinolones are associated with GI upset, tendinopathy, and photosensitivity.25
As with any antimicrobial therapy, it is worth noting that perturbing the normal vaginal flora may result in a yeast infection, especially in those patients who are prone to such infections. Anecdotal evidence suggests taking probiotics during the treatment course may help avoid yeast infections and this is often done in practice; however, data to support this is lacking. Lactobacilli strains such as Lactobacillus acidophilus have shown promise for improvement of vaginal symptoms and redness in patients with yeast infections.26
Counseling for Prevention
The most effective measure for the prevention of PID is prevention of STDs.27 To reduce the risk of STDs, patients should be encouraged to use latex condoms and receive routine screening for STDs, including HIV.1 Due to the possibility of severe complications from PID, female patients should be evaluated by an OB/GYN at least annually. Women should be counseled on the signs and symptoms of both STDs and PID, educated about avoidance of high-risk behaviors, and advised of the benefits of consistent condom use.4 Screening women for and treating cervical C trachomatis infection can reduce a woman’s risk of PID by approximately 30% to 50% over 1 year.28 If a female has been diagnosed with PID, she should be encouraged to abstain from sexual intercourse until she and her partner(s) have completed treatment.1,2,11
Sexual partners of women with PID should be screened for STDs, including partners from the previous 2 months. Treatment should cover both chlamydia and gonorrhea. If the last sexual encounter was >60 days before diagnosis, the most recent sexual partner should be screened. Education of women, especially adolescents, is critical. Discussion should include prevalence of asymptomatic infections and how PID episodes can result from future infections. Screening for reinfection should occur in 3 to 6 months.5
Vaccine development for the STD pathogens that ultimately cause PID has remained challenging. There are unique and complex immunologic characteristics of the male and female reproductive tracts, making them distinct from other mucosal tissues. Antibodies alone are not protective, only neutralizing infections. Despite challenges in previous vaccines against pathogens such as Treponema pallidum, C trachomatis, and N gonorrhoeae, new technologies provide hope for development of safe and effective vaccines against STDs, ultimately decreasing PID infection rates as well.27-29
Conclusion
PID remains a challenging condition to identify and treat due to high prevalence of asymptomatic infections. Screening for chlamydia and gonorrhea in all sexually active females younger than 25 years and in older women with high-risk behaviors is an important strategy in identifying patients with or at risk for PID and initiating early treatment. PID should be part of the differential diagnosis in all women aged 15 to 44 years with nonspecific abdominal pain. While antimicrobial therapy is highly effective, the focus of practitioners’ efforts should be to prevent long-term reproductive sequelae associated with PID. When pharmacists are involved in provision of treatment therapies for PID, it is important to discuss appropriate administration, duration of use, and common adverse effects associated with therapies.
REFERENCES
1. CDC. Pelvic inflammatory disease—CDC fact sheet (detailed). www.cdc.gov/std/pid/stdfact-pid-detailed.htm#ref8. Accessed April 11, 2016.
2. CDC. STD curriculum for clinical educators. Pelvic inflammatory disease (PID) module. October 2014. www2a.cdc.gov/stdtraining/ready-to-use/Manuals/PID/pid-notes-2014.pdf. Accessed April 13, 2016.
3. Viberga I, Odlind V, Lazdane G, et al. Microbiology profile in women with pelvic inflammatory disease in relation to IUD use. Infect Dis Obstet Gynecol. 2005;13(4):183-190.
4. Carr S, Espey E. Intrauterine devices and pelvic inflammatory disease among adolescents. J Adolesc Health. 2013;52(4 suppl):S22-S28.
5. Greydanus DE, Dodich C. Pelvic inflammatory disease in the adolescent: a poignant, perplexing, and potentially preventable problem for patients and physicians. Curr Opin Pediatr. 2015;27(1):92-99.
6. Korzeniewski K, Juszczak D. Travel-related sexually transmitted infections. Int Marit Health. 2015;66(4):238-246.
7. Hay PE, Kerry SR, Normansell R, et al. Which sexually active young female students are most at risk of pelvic inflammatory disease? A prospective study. Sex Transm Infect. 2016;92:63-66.
8. Caddy S, Yudin MH, Hakim J, Money DM. Best practice to minimize risk of infection with intrauterine device insertion. J Obstet Gynaecol Can. 2014;36(3):266-274.
9. Gradison M. Pelvic inflammatory disease. Am Fam Physician. 2012;85(8):791-796.
10. Rohrbeck P. Pelvic inflammatory disease among female recruit trainees, active component, U.S. Armed Forces, 2002-2012. MSMR. 2013;20(9):15-18.
11. Workowski KA, Bolan GA; CDC. Sexually transmitted diseases treatment guidelines, 2015. Pelvic inflammatory disease. MMWR Morb Mortal Wkly Rep. 2015;64(RR3):78-82. www.cdc.gov/mmwr/pdf/rr/rr6403.pdf. Accessed April 13, 2016.
12. Viberga I, Odlind V, Lazdane G, et al. Microbiology profile in women with pelvic inflammatory disease in relation to IUD use. Infect Dis Obstet Gynecol. 2005;13(4):183-190.
13. Sharma H, Tal R, Clark NA, Segars JH. Microbiota and pelvic inflammatory disease. Semin Reprod Med. 2014;32(1):43-49.
14. Taylor BD, Darville T, Haggerty CL. Does bacterial vaginosis cause pelvic inflammatory disease? Sex Transm Dis. 2013;40(2):117-122.
15. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929-937.
16. Sweet RL. Treatment of acute pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2011;2011:561909.
17. Smith KJ, Ness RB, Wiesenfeld HC, et al. Cost-effectiveness of alternative outpatient pelvic inflammatory disease treatment strategies. Sex Transm Dis. 2007;34:960-966.
18. Kirkclady RD, Bolan GA, Wasserheit JN. Cephalosporin-resistant gonorrhea in North America. JAMA. 2013;390:185-187.
19. Horner P, Blee K, Adams E. Time to manage Mycoplasma genitalium as an STI: but not with azithromycin 1g! Curr Opin Infect Dis. 2014;27:68-74.
20. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2015;60:1228-1236.
21. Sena AC, Lensing S, Rompalo A, et al. Chlamydia trachomatis, Mycoplasma genitalium, and Trichomonas vaginalis infections in men with nongonococcal urethritis: predictors and persistence after therapy. J Infect Dis. 2012;206(3):357-365.
22. Tepper NK, Steenland MW, Gaffield ME, et al. Retention of intrauterine devices in women who acquire pelvic inflammatory disease: a systematic review. Contraception. 2013;87(5):655-660.
23. Dhasmana D, Hathorn E, McGrath R, et al. The effectiveness of nonsteroidal anti-inflammatory agents in the treatment of pelvic inflammatory disease: a systematic review. Syst Rev. 2014;3:79.
24. Brunham R, Gottlieb S, Paavonen J. Pelvic inflammatory disease. N Engl J Med. 2015;372(21):2039-2048.
25. Lexi-Drugs. Lexi-Comp Online. Hudson, Ohio: Lexi-Comp, Inc. http://online.lexi.com. Accessed April 14, 2016.
26. Williams NT. Probiotics. Am J Health Syst Pharm. 2010;67(6):449-458.
27. Fruth U, Deal C, Dodet B, Meheus A. Vaccines for sexually transmitted infection: past, present, and future. Vaccine. 2014;32(14):1525-1526.
28. Hafner LM, Wilson DP, Timms P. Development status and future prospects for a vaccine against Chlamydia trachomatis infection. Vaccine. 2014;32(14):1563-1571.
29. Brotman RM, Ravel J, Bavoil PM, et al. Microbiome, sex hormones, and immune responses in the reproductive tract: challenges for vaccine development against sexually transmitted infections. Vaccine. 2014;32(14):1543-1552.
To comment on this article, contact rdavidson@uspharmacist.com.





