US Pharm. 2020;45(3):20-23.
ABSTRACT: Cannabidiol (CBD) is becoming more prevalent, and pharmacists must be knowledgeable about these products in order to counsel patients effectively. CBD laws and regulations are determined at the state level in the United States. Non–FDA-approved CBD products are not regulated and may contain harmful chemicals. Pharmacists must counsel patients on where and how to obtain products and to check the amount of CBD and delta-9-tetrahydrocannabinol in the product. CBD has numerous drug interactions that should be evaluated by a pharmacist. CBD is most promising for treatment-resistant seizures, and more research is necessary to evaluate its use for other indications. Sativex is currently being investigated in the U.S. for treatment of spasticity associated with multiple sclerosis and schizophrenia. In general, more studies of CBD are needed.
Cannabidiol (CBD) is gaining popularity across the United States. Pharmacists must be able to answer patients’ questions about CBD and make recommendations. This article will provide specific information about CBD, including laws, how to select a non–FDA-approved CBD product, indications for use, side effects and warnings, drug interactions, dosing and directions, pharmacokinetics, and the future of CBD oil. After reading this article, pharmacists should feel confident about counseling patients about CBD and recommending CBD products.
Laws Concerning CBD
CBD was first isolated from the Cannabis sativa plant in the 1930s. CBD is a nonpsychoactive part of the plant, whereas delta-9-tetrahydrocannabinol (THC) is the major psychoactive part of the plant. In the 1970s, researchers evaluated CBD as a pharmacologic agent.1 Epidiolex, a 100 mg/mL oral solution with less than 0.01% THC, became the first FDA-approved CBD-containing drug in June 2018.2 The drug is Schedule V and indicated only as an anticonvulsant for Lennox-Gastaut syndrome or Dravet syndrome in patients aged 2 years and older.3
In December 2018, the Agriculture Improvement Act, which removed hemp from Drug Enforcement Administration (DEA) regulation as a controlled substance, was passed and signed into U.S. law. Hemp is defined as a cannabis plant that contains no more than 0.3% THC. (In contrast, marijuana has a higher THC.) Hemp is now regulated by the U.S. Department of Agriculture and is legal in all 50 states. Laws and restrictions regarding the selling of hemp products vary by state, making it questionable to travel with CBD products.2
U.S. laws and regulations concerning CBD are determined at the state level. Currently, 33 states have legalized CBD use for medical purposes, and 10 states (Alaska, California, Colorado, Maine, Massachusetts, Michigan, Nevada, Oregon, Vermont, and Washington) and the District of Columbia have legalized marijuana for recreational use. In states such as New York, Minnesota, and Connecticut, pharmacists are required to dispense the products in authorized dispensaries. Marijuana-derived CBD oil is still considered illegal under the Controlled Substances Act in accordance with the DEA’s classification of marijuana as a Schedule I substance.2
Selecting a Non–FDA-Approved CBD Product
Pharmacists must educate patients about how to select an appropriate non–FDA-approved CBD product. These products are not tested for safety, efficacy, or quality.4 The main concerns in picking a non–FDA-approved CBD product are that it may contain harmful chemicals and may not accurately list the correct amounts of CBD and THC it contains. These products could contain harmful contaminants (e.g., pesticides, heavy metals) or have high levels of THC, which would result in a positive urine drug test.5 The patient should be advised to obtain CBD products from a medical dispensary because these products are regulated. The patient should also consider ordering products from states where CBD is legal because more testing is done in those states. When selecting a product, the patient should check the label to see if it lists the amount of CBD in each dose.5,6 The manufacturer should provide a Certificate of Analysis, which shows an independent laboratory’s assessment of the product’s potency and the presence of contaminants.5 When assessing quality, the patient should look for the Hemp Authority seal, which means that the product is legal and the manufacturer is adhering to quality standards.7
Indications for CBD
As consumer demand in the U.S. has risen, along with the number of dispensaries, the number of studies addressing the therapeutic effects of CBD has increased. The studies performed, however, are insufficient; large randomized, placebo-controlled trials need to be conducted. CBD seems most promising for treatment-resistant seizures. There is limited evidence concerning the use of CBD for psychotic symptoms in Parkinson’s disease and for anxiety related to public speaking. CBD has not been proven effective for pain, nausea, or depression.4 THC, conversely, is thought to be effective for these conditions because it has a different mechanism of action. THC activates the CB1 and CB2 receptors in the brain, and CBD does not. As mentioned previously, CBD does not have psychotropic effects and THC does. These differences are believed to account for the different uses of CBD and THC.7,8
Patients with early-onset epilepsy who are resistant to conventional therapy may benefit from CBD oil. A trial that investigated the effect of CBD on drop seizures of Lennox-Gastaut syndrome found that CBD 10 mg/kg/day or 20 mg/kg/day, when added to conventional therapy, led to a greater reduction of drop seizures compared with placebo.9 The most common adverse reactions were somnolence, decreased appetite, and diarrhea. Specific adverse events from CBD included elevated liver aminotransferase concentrations.9
Clinical findings on the use of CBD oil in Parkinson’s disease (PD) remain unclear. Past studies have evaluated CBD’s efficacy in minimizing nonmotor symptoms of PD, such as cognitive deficits, sleep disturbances, psychosis, depression, and anxiety.10 The neuroprotective properties of CBD have been studied in animals with PD, with results indicating that CBD appears to reduce psychotic symptoms.11 Although patients with PD have reported fewer sleep disturbances as well as improvements in quality of life, treatment in humans requires further investigation on a larger scale, with longer durations and more standardized dosing.12 Most studies have used combinations of CBD and THC extracts, including nabilone, a synthetic CB1 receptor agonist. CBD dosages of 150 mg/day for 4 weeks and titrated by 140 mg/week were found to be safe and well tolerated and did not worsen motor function.10
More evidence is needed to support the use of CBD for anxiety. Studies have found that CBD 300 mg may be effective for anxiety related to public speaking, and doses of 400 mg to 600 mg may help patients with social anxiety disorder and public speaking–related anxiety. Studies are inconclusive concerning the utility of CBD for anxiety.13
Side Effects and Warnings
Studies have reported various properties and potential benefits of CBD. Some undesired side effects of CBD use are decreased appetite, dry mouth, diarrhea, dizziness, drowsiness, fatigue, lightheadedness, orthostatic hypotension, psychomotor slowing, sedation, somnolence, weight loss, and increased risk of liver injury with dosages of 20 mg/kg/day or the use of clobazam or valproate. Monitoring of liver enzymes, weight, and cognitive function may be warranted. CBD can pass through the placenta, so it is recommended that CBD be avoided during pregnancy and while breastfeeding. Because CBD oils may contain trace amounts of THC, operating heavy machinery and driving should be avoided when treatment is initiated.1
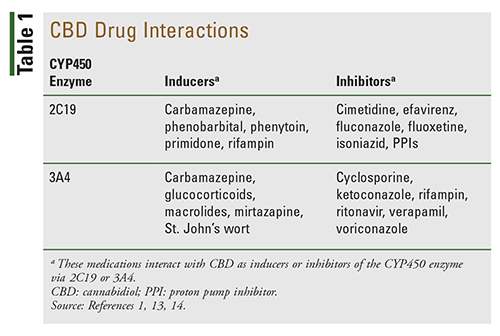
Drug Interactions
CBD is metabolized in the liver, mainly by CYP2C19, CYP3A4, and UGT. This can lead to interactions with prescription drugs, OTC medications, and herbal supplements.1,14
The inhibition of CYP2C19 by CBD can increase levels of carisoprodol, citalopram, clopidogrel, diazepam, phenytoin, proton pump inhibitors (PPIs), valproic acid, and warfarin. As a strong CYP3A4 inducer, CBD may lessen the efficacy of amlodipine, atorvastatin, buprenorphine, bupropion, diltiazem, eplerenone, fentanyl, loperamide, midazolam, paclitaxel, pioglitazone, sildenafil, solifenacin, tamsulosin, testosterone, topiramate, zolpidem, and other 3A4 substrates.7
More serious effects may occur with concomitant use of central nervous system depressants, such as barbiturates, benzodiazepines, fentanyl, morphine, and propofol. These effects are the result of the synergistic effects of sedation and hypnotic effects at high doses. Increased sedative effects may also be seen with herbal supplements, including kava, melatonin, S-adenosylmethionine, and St. John’s wort.13,14
Other interactions to be aware of are presented in TABLE 1.
Dosing and Directions
In unregulated dispensaries, CBD oil sold comes in a sublingual formulation known as CBD tincture and is generally available in 30-mL bottles with dropper caps.15 A bottle costs approximately $20. The concentration of the tincture ranges from about 1,500 mg to 3,000 mg per bottle. If a drop equals 0.05 mL, one bottle contains approximately 600 drops of CBD oil. Drops are usually placed under the tongue, and the patient should let the oil absorb into the lining of the mouth, without swallowing, for 30 seconds to 1 minute. Capsules and gummies are also available.15
Epidiolex Dosing
As noted earlier, Epidiolex (CBD) is an FDA-approved oral solution for treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome. The cost of Epidiolex is approximately $2,708 per month. It is supplied as 100 mL of solution containing CBD 100 mg/mL. For both indications, the initial starting dosage is 2.5 mg/kg orally twice daily for 1 week. The dosage may be titrated weekly in increments of 2.5 mg/kg twice daily to a maintenance dosage of 5 mg/kg twice daily. The maximum dosage is 10 mg/kg twice daily or 20 mg/kg/day. Gradual tapering is recommended when Epidiolex is discontinued.3
Starting at a low dosage is recommended for elderly patients and patients with moderate or severe hepatic impairment. The dosage should be 1.25 mg/kg to 5 mg/kg twice daily or 0.5 mg/kg to 2 mg/kg twice daily, respectively.3
Pharmacokinetics
CBD reaches its maximum concentration in 2.5 to 5 hours. High-calorie and high-fat meals can increase the maximum concentration of drug fivefold and the AUC fourfold.14 Owing to the first-pass effect, CBD is poorly absorbed, with a bioavailability of 13% to 19%. Better bioavailability has been reported with inhaled CBD (11% to 45%). CBD is 94% protein bound; therefore, interactions may occur with other highly protein bound drugs or in patients who have abnormal albumin levels. The volume of distribution is 20,963 L to 42,849 L, meaning that the drug is largely distributed into the tissues. CBD is metabolized by the gut and primarily by the liver. Epidiolex has an active metabolite, 7-OH-CBD, and is a 2C19 and 3A4 substrate and inhibitor of 2C19, 1A2, UGT1A9, and UGT2B7. Its elimination half-life is 56 to 61 hours. CBD is excreted primarily in the feces and urine.3
The Future of CBD
Sativex (nabiximols) is an oromucosal spray that contains CBD and THC in a 1:1 ratio. The active ingredients are absorbed sublingually or buccally. Sativex is currently under investigation in the U.S.; however, more than 25 countries worldwide have approved Sativex for the treatment of spasticity associated with multiple sclerosis. Sativex is also being researched for potential treatment of schizophrenia and other conditions.16
The Pharmacist’s Role
Some pharmacists are hesitant to get involved with CBD. Prosecution by federal law could lead to severe consequences, including fines, imprisonment, or loss of DEA registration for pharmacies, ultimately stripping them of their ability to dispense controlled substances. If U.S. laws and regulations were more uniform across states, many of the concerns surrounding CBD would be eliminated. Until then, patients must use caution when selecting a product from an unregulated source because of the possibility of contamination and product misbranding.17 Although more testing is needed, it is imperative for pharmacists to understand what to recommend to patients. Pharmacists should counsel patients on the risks and benefits of treatment. Patients who are are using CBD should be reminded to obtain the product from a reputable manufacturer.17
Conclusion
Pharmacists need to keep abreast of current information on CBD in order to assist patients who are interested in using it. While most studies are inconclusive, there currently is enough information to effectively guide patients in choosing a treatment. CBD has the most evidence for treatment-resistant seizures; other indications need further study. Patients must be counseled to choose an appropriate product from a reputable source. CBD may be misbranded or contaminated with harmful chemicals. Pharmacists are uniquely positioned to assess the numerous potential drug interactions with CBD. New prescription CBD products are currently being investigated in the U.S.
REFERENCES
1. Food, herbs & supplements: cannabidiol (CBD). Natural Medicines Database [subscription required]. https://naturalmedicines.therapeuticresearch.com/databases/food,-herbs-supplements.aspx?letter=C. Accessed February 20, 2020.
2. Is CBD oil legal? www.cbdcentral.com/is-cbd-legal. Accessed February 20, 2020.
3. Epidiolex (cannabidiol) package insert. Carlsbad, CA: Greenwich Biosciences, Inc; December 2018.
4. Demystify cannabidiol oil. Pharm Letter. September 2018.
5. Gill LL. How to shop for CBD. Consumer Reports. www.consumerreports.org/cbd/how-to-shop-for-cbd. Accessed February 20, 2020.
6. Project CBD. How do I choose a CBD product? www.projectcbd.org/how-to/10-tips-for-buying-cbd. Accessed February 20, 2020.
7. Comparison of cannabinoids. Pharm Letter/Presc Letter. September 2018.
8. VanDolah HJ, Bauer BA, Mauck KF. Clinicians’ guide to cannabidiol and hemp oils. Mayo Clin Proc. 2019;94(9):1840-1851.
9. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378(20):1888-1897.
10. Crippa JAS, Hallak JEC, Zuardi AW, et al. Is cannabidiol the ideal drug to treat non-motor Parkinson’s disease symptoms? Eur Arch Psychiatry Clin Neurosci. 2019;269(1):121-133.
11. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313(24):2474-2483.
12. Elsaid S, Kloiber S, Le Foll B. Effects of cannabidiol (CBD) in neuropsychiatric disorders: a review of pre-clinical and clinical findings. Prog Mol Biol Transl Sci. 2019;167:25-75.
13. MedLinePlus. Cannabidiol (CBD): are there interactions with medications? https://medlineplus.gov/druginfo/natural/1439.html. Accessed February 20, 2020.
14. White MC. Cannabidiol (CBD): a tale of two products. https://ce.pharmacy.uconn.edu/wp-content/uploads/sites/2102/2019/05/CBD-MAY2019-FINAL.pdf. Accessed February 20, 2020.
15. Wong C. The health benefits of CBD oil. www.verywellhealth.com/cbd-oil-benefits-uses-side-effects-4174562. Accessed February 20, 2020.
16. GW Pharmaceuticals. Sativex. www.gwpharm.com/healthcare-professionals/sativex. Accessed February 20, 2020.
17. Explain risks and benefits of medical marijuana. Pharm Letter. September 2017.
To comment on this article, contact rdavidson@uspharmacist.com.






