US Pharm. 2021;46(9):45-49.
ABSTRACT: Since Oregon became the first state allowing pharmacists to prescribe hormonal contraceptives, many other states across the country have followed suit. States vary in terms of what pharmacists are allowed to do when furnishing hormonal contraceptives based on the designated prescriptive authority. Training requirements typically entail a state board-approved program or continuing education program. General steps for a pharmacist to prescribe hormonal contraceptives include screening, counseling, documentation, and monitoring. Some states have restrictions in their protocols. Community pharmacists remain integral providers who can prescribe hormonal contraceptives within their scope of practice.
Hormonal contraceptives are one of the most effective methods of preventing unintended pregnancies. The United States has seen a decrease in pregnancy rates in the past few decades, but it is still estimated that 45% of all pregnancies are unintended.1 The healthcare world can effectively improve access and adherence to contraceptive regimens for vulnerable populations if pharmacies are able to provide contraceptives to their local communities. Vulnerable populations for unintended pregnancies include young women, uninsured individuals, and those lacking adequate health literacy. Pharmacists have an advantage in serving their communities due to their locations, extended hours compared with clinics, and no appointment requirements for consultations.1
Pharmacists gain the authority to prescribe medications to their patients through a variety of methods depending on each state’s laws and regulations. State policies can range from autonomous authority (least restrictive) to collaborative (most restrictive). Independent prescribing (including statewide protocols) falls under autonomous prescriptive authority. This is a framework that specifies the conditions that authorize pharmacists to prescribe certain medications when providing a service. The protocol dictates specific qualifications for implementation and procedures that should be followed by pharmacists. The collaborative practice agreement (CPA) is a voluntary, formal relationship between pharmacists and physicians or other providers.2
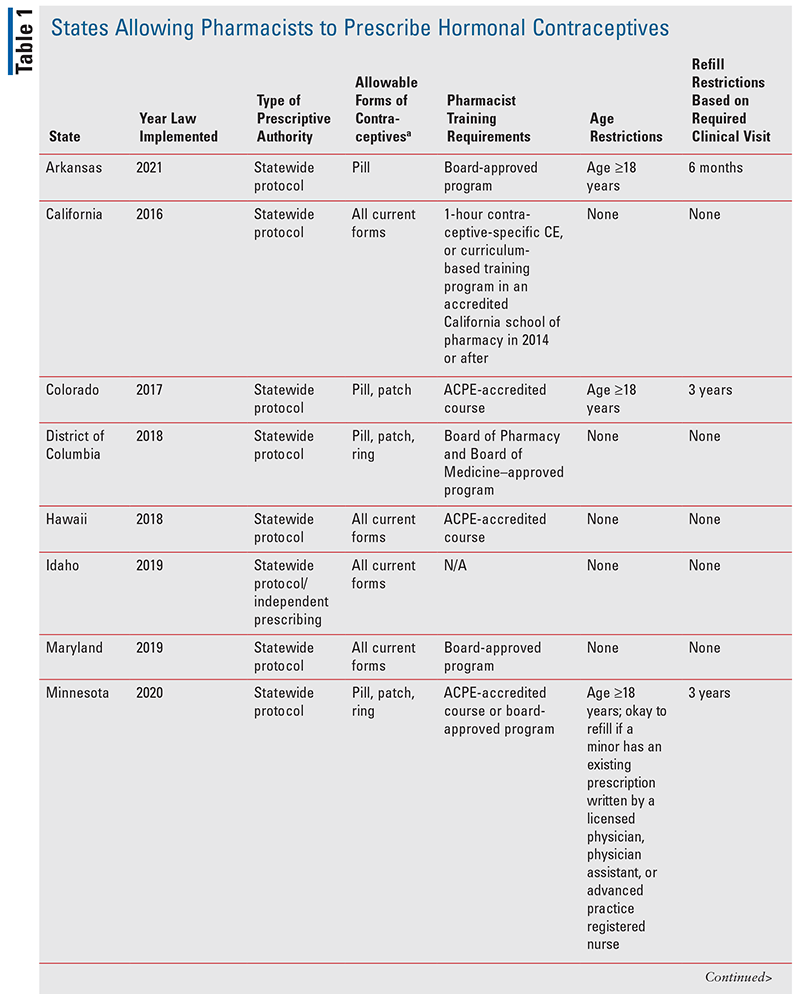
Pharmacists have previously advocated for prescribing authority for contraceptives. The first instance of acceptance occurred in 2013 with legislation in California stating that this authority would be active by 2016. In 2016, Oregon and California authorized pharmacists to prescribe hormonal contraceptives, with four more states following shortly (Colorado, New Mexico, Hawaii, and Maryland).3
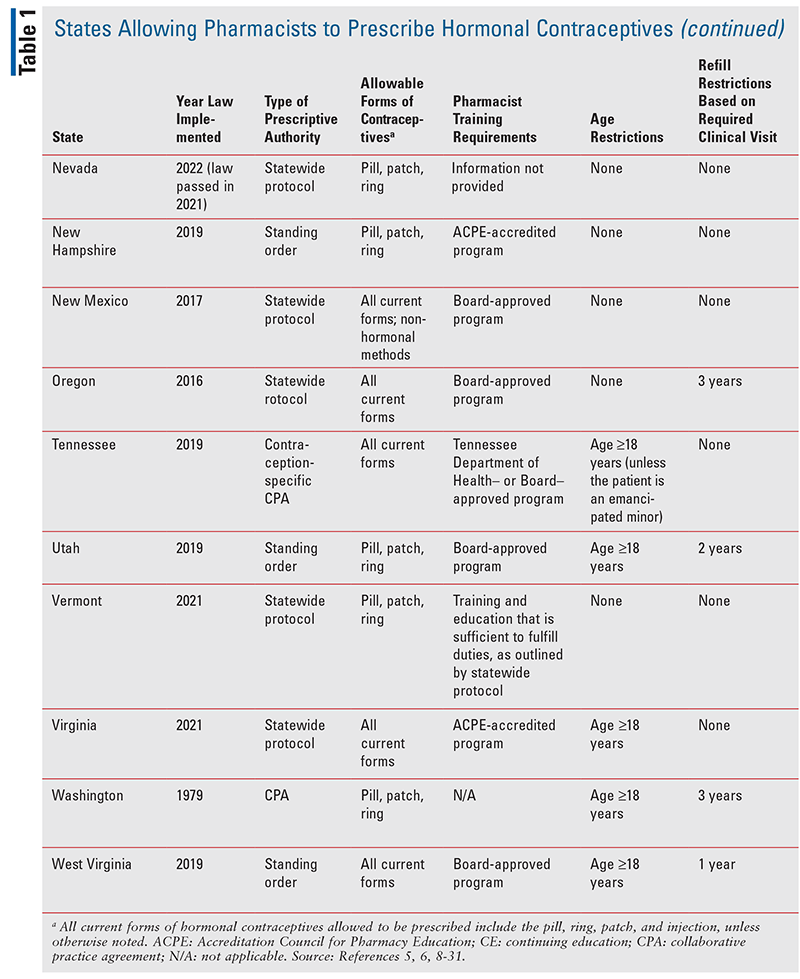
As of June 2021, 17 U.S. jurisdictions and the District of Columbia allow pharmacists to prescribe hormonal contraceptives (TABLE 1).4 Fifteen states and the District of Columbia allow the prescribing privilege through a statewide protocol or standing order; two states allow it through a CPA.5 Many other states have proposed bills that are pending legislation: Arizona, Delaware, Illinois, Indiana, Iowa, Kansas, Massachusetts, Missouri, New Jersey, New York, North Carolina, Rhode Island, South Carolina, and Wisconsin.6
Preprescribing Process
Although each state varies in terms of limitations and specific protocols, each state’s prescriptive authority includes standard processes to be followed by the pharmacist before issuing a prescription, typically via an approved algorithm (FIGURE 1).
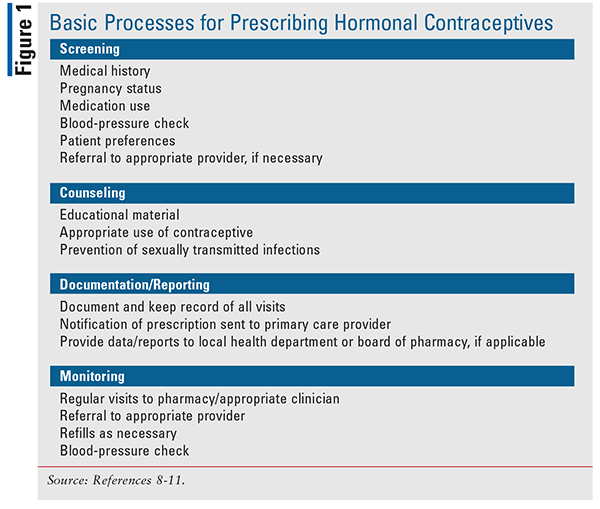
A screening questionnaire must be completed first to ensure that the patient meets eligibility criteria based on the CDC’s U.S. Medical Eligibility Criteria for Contraceptive Use.7 This helps the pharmacist to clearly determine whether a prescription will be appropriate and safe for the patient or whether the patient should instead be referred to a physician. Questions to ask the patient will include those related to medical history, pregnancy status, and medication use. Since some contraceptives are unsafe in the setting of uncontrolled hypertension, a blood-pressure check is warranted to ensure safety. If the pharmacist identifies risk factors for unsafe or ineffective use of the hormonal contraceptives that are allowed to be prescribed, a referral to the patient’s primary care physician should follow. If the patient does not have a primary care physician, the pharmacist should provide information about a local clinic and education about the importance of establishing a relationship with a primary care provider.
As with any other prescription dispensed, the pharmacist should counsel the patient about the hormonal contraceptive, how to use it properly, side effects, and the importance of adherence. Education about using backup methods is warranted based on when the patient starts their contraceptive. Education on prevention of sexually transmitted infections is also incorporated into the protocol. Additionally, the pharmacist must document the visit with the patient and provide a copy for the patient’s medical provider(s). Some states require reporting of the pharmacy’s prescriptions to be forwarded to the local health department or board of pharmacy. The patient is encouraged to return for regular blood- pressure monitoring, if necessary, or to follow up with their primary care provider.11
Challenges
Despite significant progress in legislation, there are still limitations associated with pharmacists’ prescribing of hormonal contraceptives. For example, if a patient prefers a long-acting reversible contraceptive such as an intrauterine device or implant, she would be required to seek the care of an appropriate provider, such as a gynecologist or primary care physician. If a patient does not know her complete medical history, the pharmacist may not be able to authorize a prescription. Moreover, a patient who does not regularly see a primary care provider may not be aware of underlying medical conditions, which could lead to unsafe prescribing of a contraceptive. Many community pharmacies also do not have access to a patient’s medical records and thus would not be privy to certain information that the patient may forget to disclose. Thus, a patient’s recall bias may either prevent them from receiving a contraceptive or cause harm if she receives it when she should not have.
Certain states restrict the age of patients to whom pharmacists can prescribe hormonal contraceptives; in some states, pharmacists cannot prescribe contraceptives for minors younger than age 18 years.5,6 Refills may be restricted if the patient has not seen a physician within a certain number of years as dictated by the respective state law. Although pharmacists prescribing hormonal contraceptives serve as bridges to care for patients who cannot easily access primary care, this legal restriction remains a barrier for those same patients.6 Pharmacies or pharmacists may not participate in providing such services due to the lack of reimbursement for the cognitive services. Furthermore, reimbursement may require that pharmacists submit additional paperwork to enroll as a billable provider. States are beginning to pass or amend existing laws to reimburse pharmacists for rendered services.8
Conclusion
It has been almost a decade since Oregon passed legislation allowing pharmacists to prescribe hormonal contraceptives. To date, 17 states and the District of Columbia allow pharmacists to prescribe hormonal contraceptives for women and 14 have proposed legislation pending. The pharmacists’ prescriptive authority benefits both patients and physicians; not only does it help to take a load off physicians’ busy schedules but it also addresses barriers to care for women who need the contraceptives.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Rodriguez MI, Edelman AB, Skye M, et al. Association of pharmacist prescription with dispensed duration of hormonal contraception. JAMA Netw Open. 2020;3(5):e205252.
2. National Alliance of State Pharmacy Associations. Pharmacist prescribing: statewide protocols and more. November 9, 2018. https://naspa.us/resource/swp/. Accessed July 2, 2021.
3. National Alliance of State Pharmacy Associations. Pharmacists provide access to care: contraceptive prescribing. February 26, 2018. https://naspa.us/resource/rph-access-contraceptives/. Accessed June 21, 2021.
4. National Alliance of State Pharmacy Associations. Pharmacist prescribing: hormonal contraceptives. April 22, 2021. https://naspa.us/resource/contraceptives/. Accessed June 21, 2021.
5. Joslin CM, Greenhut S. Birth control in the states: a review of efforts to expand access. R Street Policy Study No. 159. November 2018. www.rstreet.org/2018/11/21/birth-control-in-the-states-a-review-of-efforts-to-expand-access/. Accessed August 12, 2021.
6. Joslin CM. Lessons for legislators: a guide to allowing pharmacist-prescribed birth control. R Street Policy Study No. 207. September 2020. www.rstreet.org/2020/09/30/lessons-for-legislators-a-guide-to-allowing-pharmacist-prescribed-birth-control/. Accessed August 12, 2021.
7. Tepper NK, Curtis KM, Cos S, Whiteman NK. Update to U.S. medical eligibility criteria for contraceptive use: updated recommendations for the use of contraception among women at high risk of HIV infection. MMWR Recomm Rep. 2020;69:405-410.
8. Orris A, Mauser G, Bachrach D, Craven M. Implementing pharmacist contraceptive prescribing: a playbook for states and stakeholders. Manatt Health. January 2021. www.manatt.com/Manatt/media/Documents/Articles/Implementing-Pharmacist-Contraceptive-Prescribing_v3.pdf. Accessed August 12, 2021.
9. Power to Decide. State reproductive health access policies. https://powertodecide.org/what-we-do/access/state-policy/rh-access-policies/pharmacist-prescribing/ID. Accessed August 10, 2021.
10. Birth Control Pharmacist. Policies: pharmacist prescribing of hormonal contraception. January 18, 2019. https://birthcontrolpharmacist.com/policies/. Accessed June 25, 2021.
11. Rafie S, Landau S. Opening new doors to birth control: state efforts to expand access to contraception in community pharmacies. Birth Control Pharmacist. 2019. https://birthcontrolpharmacist.com/2019/12/21/report/. Accessed August 10, 2021.
12. Kellogg S. Senate committee passes bill allowing Arkansas pharmacists to provide birth control. Ualrpublicradio.org. March 16, 2021. www.ualrpublicradio.org/post/senate-committee-passes-bill-allowing-arkansas-pharmacists-provide-birth-control. Accessed August 10, 2021.
13. Arkansas State Legislature. 93rd General Assembly—Regular session 2021 HB 1069—To amend the provisions of the Arkansas code concerning the practice of pharmacy; and to authorize pharmacists to provide access to and administration of oral contraceptives. www.arkleg.state.ar.us/Bills/Detail?tbType=&id=hb1069&ddBienniumSession=2021%2F2021R. Accessed August 11, 2021.
14. California State Legislature. Senate Bill No. 493. Chapter 469, 2013. An act to amend Sections 733, 4040, 4050, 4051, 4052, 4052.3, 4060, 4076, 4111, and 4174 of, and to add Sections 4016.5, 4052.6, 4052.8, 4052.9, 4210, and 4233 to, the Business and Professions Code, relating to pharmacy. https://leginfo.legislature.ca.gov/faces/billPdf.xhtml?bill_id=201320140SB493&version=20130SB49390CHP. Accessed August 11, 2021.
15. State of Colorado Department of Regulatory Agencies. Colorado State Board of Pharmacy approved statewide protocol for prescribing hormonal contraceptive patches and oral contraceptives. https://drive.google.com/file/d/0B-K5DhxXxJZbNl80aFVwWjJmbk0/view?resourcekey=0-TJH2ilWE9c8billMeOqjlg. Accessed June 26, 2021.
16. Code of the Disttict of Columbia § 3–1202.08. Board of pharmacy and advisory committee on clinical laboratory practitioners. https://code.dccouncil.us/dc/council/code/sections/3-1202.08.html. Accessed June 26, 2021.
17. Hawaii State Legislature. SB 513. Pharmacists; prescriptive authority; contraceptive supplies; reimbursement. 29th Legislature 2017. www.capitol.hawaii.gov/Archives/measure_indiv_Archives.aspx?billtype=SB&billnumber=513&year=2017. Accessed August 11, 2021.
18. Idaho Legislature. House Bill 182, Pharmacists–amends existing law to revise provisions regarding products that may be prescribed. Idaho 2019. https://legislature.idaho.gov/sessioninfo/2019/legislation/h0182/. Accessed August 11, 2021.
19. Maryland Department of Health. Code of Maryland Regulations. Chapter 10.34.40—Pharmacists Prescribing Contraceptives. http://mdrules.elaws.us/comar/10.34.40. Accessed August 11, 2021.
20. Minnesota Board of Pharmacy. Pharmacist prescribing protocol self-administered hormonal contraceptives. Last updated December 24, 2020. https://mn.gov/boards/assets/Minnesota%20Board%20of%20Pharmacy%20Protocol%20for%20Pharmacist%20Contraceptive%20Prescribing%20Approved_tcm21-464043.pdf. Accessed June 30, 2021.
21. Nevada Legislature. SB 190, 2021 Legislature, 81st Session. An act relating to contraceptives. www.leg.state.nv.us/App/NELIS/REL/81st2021/Bill/7622/Overview. Accessed August 11, 2021.
22. New Hampshire House Bill. 1822, 2018 Legislature, Regular Session. Making hormonal contraceptives available directly from pharmacists by means of a standing order. https://legiscan.com/NH/text/HB1822/2018. Accessed August 11, 2021.
23. New Mexico Regulation and Licensing Department. Protocol for pharmacist prescription of hormonal contraception. www.rld.state.nm.us/uploads/files/OCProtocolApproved(1).pdf. Accessed June 30, 2021.
24. Oregon Legislature. H.B. 2879, 2015 Legislature, Regular Session. Permits pharmacists to prescribe hormonal contraceptive patches and self-administered oral hormonal contraceptives. https://gov.oregonlive.com/bill/2015/HB2879/. Accessed August 11, 2021.
25. Tennessee Code Chapter 1140-15. Prescribing and dispensing of hormonal contraceptives. Tenn. Code Ann. § 63-10-219 (2018). https://publications.tnsosfiles.com/rules/1140/1140-15.20180718.pdf. Accessed June 30, 2021.
26. Utah Department of Health. Utah statewide standing order pharmacist dispensing of hormonal contraception. https://health.utah.gov/wp-content/uploads/ContraceptiveStandingOrderFINAL.pdf. Accessed June 26, 2021.
27. Vermont Legislature. Act No. 157 (H.663). Health; contraceptives; health insurance; schools (2020). https://legislature.vermont.gov/Documents/2020/Docs/ACTS/ACT157/ACT157%20As%20Enacted.pdf. Accessed June 30, 2021.
28. Vermont Secretary of State, Office of Professional Regulation. Vermont pharmacist prescribing protocol–self-administered hormonal contraceptives. pharmacy statutes, rules & resources. June 21, 2021. sos.vermont.gov/media/bj0hpx4r/vermont-pharmacist-prescribing-protocol-self-administered-hormonal-contraceptives.pdf. Accessed July 3, 2021.
29. Virginia Department of Health Professions. Pharmacist routine contraceptive statewide protocol. September 9, 2020. www.dhp.virginia.gov/pharmacy/docs/protocols/Pharmacist%20routine%20contraceptive%20statewide%20protocol-9-9-2020.docx. Accessed August 11, 2021.
30. Washington Laws 1979. Chapter 90. Practice of Pharmacy—Requirements. https://leg.wa.gov/CodeReviser/documents/sessionlaw/1979c90.pdf?cite=1979%20c%2090%20%C2%A7%205. Accessed June 30, 2021.
31. West Virginia Legislature. House Bill 2583, 2019 Legislature Regular Session. Family Planning Access Act. www.wvlegislature.gov/Bill_Status/bills_text.cfm?billdoc=hb2583%20intr.htm&yr=2019&sesstype=RS&i=2583. Accessed August 11, 2021.
To comment on this article, contact rdavidson@uspharmacist.com.