US Pharm. 2012;37(9):HS2-HS6.
Perhaps the greatest challenge facing health care providers when administering pharmacologic agents during labor and delivery is that most of the drugs are used off label.1 Furthermore, when selecting appropriate therapy, health care providers need to consider the effect of the medication on both the mother and the fetus. It is therefore imperative that providers be conversant with the various options available and their associated restrictions. This article discusses the medications that are commonly used during labor and delivery, namely for cervical ripening, labor induction, and labor pain management.1
Induction and Augmentation of Labor
The goal of labor induction and augmentation at term is to facilitate vaginal delivery of a healthy infant.2 A normal uterus is spontaneously contractile, but it changes during pregnancy to maintain a quiescent state and a firm status to retain the fetus.3 In the final 4 to 5 weeks of pregnancy, endogenous prostaglandins are released to: 1) ripen the cervix, making it soft and dilated, and 2) sensitize the uterus in preparation for labor.4
Pregnancies that go beyond term, pregnancies in which premature rupture of the membranes occurs, or pregnancies in which there are maternal or fetal indications for early delivery commonly require induction of labor.3 The rate of induced labor ranges from 9.5% to 33.7% of all pregnancies annually.5 Various drugs can be used to induce and augment labor, but they are commonly associated with either the inability to achieve effective labor or uterine hyperstimulation.4 In some cases, women may be offered sweeping of the membranes before drug therapy is considered, but this can increase maternal discomfort, irregular contractions, and bleeding.3
Cervical ripening can be assessed using the Bishop score, a measure that incorporates a number of factors including the position, consistency, thickness, and dilation of the cervix as well as the position of the fetus.5 As a rule of thumb, a patient with a Bishop score of over 8 is likely to achieve a successful vaginal birth; a patient with a Bishop score of less than 6 is likely to require a cervical ripening agent.
Nonpharmacologic cervical ripening methods have been used for centuries, although there are not many studies to prove their efficacy. These include herbal compounds, castor oil, hot baths, enemas, sexual intercourse, breast stimulation, acupuncture, acupressure, transcutaneous nerve stimulation, and mechanical and surgical modalities.5
Drug Therapy for Cervical Ripening
During cervical ripening, the collagen fiber alignment, the collagen fiber strength, and the tensile strength of the extracellular cervical matrix decrease and the cervical dermatan sulfate proteoglycan 2 levels increase. The collagen fibers separate and the cervix softens.6 These processes are targeted by pharmacologic cervical ripening agents such as prostaglandins, prostaglandin analogues, and oxytocin.
Prostaglandins: Prostaglandins are released as a natural part of the cervical ripening processes. Both F- and E-series prostaglandins allow for an increase in intracellular calcium levels causing the contraction of myometrial muscle.7 Specifically, prostaglandin F2-alpha causes an increase in the glycosaminoglycans. The E-series prostaglandins tend to be more uteroselective and primarily cause7:
- Dilation of small vessels in the cervix
- Increase in collagen degradation
- Increase in hyaluronic acid, elastase, and dermatan sulfate5
- Increase in chemotaxis for leukocytes, which causes increased collagen degradation
- Increase in stimulation of interleukin (IL)-8 release.
A recent study has shown that the use of intravaginal prostaglandins increases the likelihood that a vaginal delivery will occur within 24 hours compared to placebo, which had no increase in operative delivery rates.8 Intravenous prostaglandins, on the other hand, do not show an advantage but produce increased maternal side effects compared to other methods of induction.9,10 More recently, researchers have studied the benefits of using a combination of a mechanical method (i.e., the Foley catheter) and a pharmacologic method (i.e., prostaglandins or “extra-amniotic prostaglandins”), but there are insufficient data to recommend this method at present.9 Prostaglandins are associated with a number of risks including uterine hyperstimulation and maternal nausea, vomiting, diarrhea, and fever.
Dinoprostone is the only prostaglandin currently available for cervical ripening in the form of both a vaginal gel (Prepidil 0.5 mg) and a vaginal insert (Cervidil 10 mg). The gel needs be kept refrigerated and brought to room temperature before use and should only be administered with the patient near a delivery suite. Patients using the gel need to be kept in the recumbent position for the first 30 minutes and should be monitored for a further 30 to 120 minutes. Contractions typically appear within 60 minutes and peak within 4 hours. The cervix is said to be ready when strong uterine contractions occur, the Bishop score is 8 or more, or a change in maternal or fetal status is noted.10 The inserts provide a lower constant release of medication than the gel dose, with a similar efficacy. They do not require refrigeration and are easier to remove in cases of hyperstimulation.
Prostaglandin Analogues: Misoprostol (Cytotec) is a synthetic prostaglandin (PGE1) analogue that is a safe and inexpensive option for cervical ripening, albeit an unlicensed indication. It can be administered orally at a dose of 50 to 100 mcg that may be repeated up to every 4 hours. Peak concentrations are achieved at 12 minutes following absorption and de-esterification to the active free acid, with a half-life of 21 minutes. Vaginally, a 25-mcg insert can be given every 3 to 6 hours up to a maximum of 50 mcg per dose. Misoprostol is three times more bioavailable when administered vaginally than the oral formulation.10
While misoprostol is associated with an increased incidence of tachysystole, current data show that the number of patients requiring cesarean section because of fetal heart rate (FHR) abnormalities, the need for terbutaline administration to arrest labor, and the frequency of meconium aspiration syndrome is not significantly different from patients given oxytocin or dinoprostone.10 Uncommon side effects of misoprostol use include hypertonus and hyperstimulation syndrome, uterine rupture, fetal demise, nausea, and vomiting.10
Oxytocin: As a pregnancy progresses, the number of oxytocin receptors increases, the phospholipase C–inositol pathway is activated, and intracellular calcium levels increase, stimulating contractions in myometrial smooth muscle. Used since the 1950s, oxytocin is still the preferred pharmacologic agent for cervical ripening or inducing labor once the cervix is favorable.10 It can also be used for labor induction after misoprostol is administered for cervical ripening.
An infusion of no more than 4 mU per minute is effective for cervical ripening and produces relatively few side effects. Spontaneous labor normally occurs in 8 to 12 hours. One study found that 50 mcg of intravaginal misoprostol before an oxytocin infusion is more effective than oxytocin alone for cervical ripening.11 Oxytocin has been shown to increase the chances of vaginal birth, although it is generally less effective than prostaglandins in this regard.12 It is potent but generally well tolerated, easy to titrate, and has a short half-life of about 1 to 5 minutes. However, dose-related adverse effects have been observed, including an antidiuretic effect in high doses. Other side effects include uterine hyperstimulation and uterine rupture. Continuous FHR monitoring is required; if the FHR falls, the oxytocin dose can be lowered rather than completely stopped.3
Other Agents: Relaxin, mifepristone, corticosteroids, estrogens, hyaluronidase, and isosorbide mononitrate are all currently under investigation for cervical ripening and inducing labor.9,13,14
Pain Management
While pain relief during labor is a well-documented controversy, pharmacologic agents are widely used in obstetrics. A painful labor may not only produce maternal physiological and biochemical changes, but could also have adverse fetal effects.15 The pain may result in maternal anxiety, which is associated with an increase in plasma catecholamines and cortisol and an increase in the length of labor. The final result of these reactions has been associated with maternal and fetal metabolic acidosis.15
Labor is generally divided into three stages, and the pain associated with each stage has its own source.16 Beginning from the start of regular uterine contractions until the completion of cervical dilatation, the first stage is associated with visceral pain and is due to the contractions and dilation of the uterine segment and cervix. The second stage leads on from this point up to the completion of delivery, and the pain is primarily somatic as a result of the fetus in the birth canal, causing distention and tearing of the vaginal and perineal tissues.17 The third stage of delivery is the postpartum stage.
Some women may choose to use breathing and mental exercises to control the pain during labor. There is insufficient evidence on the efficacy of these methods, and women may opt for pharmacologic pain relievers, including nerve blocks and systemic analgesia.17,18 In the last decade, the prescription use of opioids and nonopioid analgesics for pain management during pregnancy has increased by about 40%.19 The workforce survey conducted in 2001 showed an increase in the use of epidural analgesia.20 Both spinal analgesia and combined spinal analgesia were used in a small number of maternity cases, and a slightly larger proportion of patients used patient-controlled analgesia.
Systemic Analgesia
Nitric Oxide: Inhaled nitric oxide is a fairly effective and relatively safe option (for the mother and fetus) for analgesia.17 It does not interfere with the release and function of endogenous oxytocin, and has no adverse effects on the normal physiology and progress of labor.21 Patients may experience nausea, vomiting, and dizziness.18 Since nitric oxide is rapidly excreted by the lungs of the newborn, it is relatively safe even though it crosses the placenta readily.15 Patients must be monitored for hypoxemia if nitric oxide is used together with systemic opioids.17 A recent survey, however, identified only two centers in the United States where it is routinely available, and it was used by only about 1% of women, perhaps because its efficacy has not been established.22
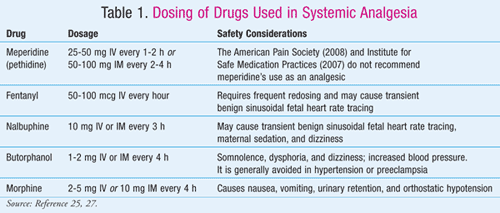
Opioid Agonists and Antagonists: All the drugs in this group share similar pharmacologic profiles but differ in potency, pharmacokinetics, and side effects (TABLE 1).23 Opioid agonists and antagonists provide systemic analgesia that is useful over neuraxial analgesia when17,24:
- Neuraxial analgesia is contraindicated, e.g., in pre-existing coagulopathy, infection at puncture site, hemorrhage, hypovolemia of other cause, untreated systemic infection, preload-dependent disease states, and lumbar spinal pathology
- Neuraxial analgesia is refused, or not needed
- A skilled anesthesia provider is not available.
However, while systemic analgesia is widely used, it lacks rigorous scientific evidence, and the analgesic effect achieved is incomplete. Furthermore, the drugs in this group cross the placenta and cause neonatal as well as maternal side effects.17
The common side effects of opioids include maternal hypotension, increased maternal temperature, postdural puncture headache, transient fetal heart deceleration, pruritus, and maternal nausea, vomiting, and drowsiness.25,26 Upon delivery, the newborn may experience neonatal depression and FHR abnormalities, as well accumulation of the drug in its system.
Neuraxial Analgesia
Labor pain is transmitted through lower thoracic, lumbar, and sacral roots that can be blocked by an epidural, a spinal, or a combined spinal epidural (CSE). A spinal block is a single shot of a long-acting anesthetic administered into the thecae of the spine with or without an opioid. It is commonly used for the second stage of labor or a cesarean. An epidural is a combination of a local anesthetic and an opioid injected into the epidural space through a catheter to allow for continuous infusion. Doses can be titrated through the entire labor, and the same catheter can be used for labor, vaginal delivery, and a cesarean. Block via an epidural is not usually initiated until active labor is established. A CSE combines the rapid onset of spinal analgesia with the continuous infusion of an epidural. CSEs are widely used to provide immediate relief during the second stage of labor and can convert to adequate analgesia if a cesarean is required.27
Neuraxial analgesia provides superior pain relief over systemic methods (TABLE 2).25,28 Neuraxial labor analgesia often combines opioids as well as local anesthetics that work synergistically and allow the use of lower doses of each agent, thereby minimizing side effects.17 The opioids are useful for the visceral pain of early stage 1 labor, and the anesthetics are useful for managing the somatic pain of late stage 1 and stage 2 labor.17
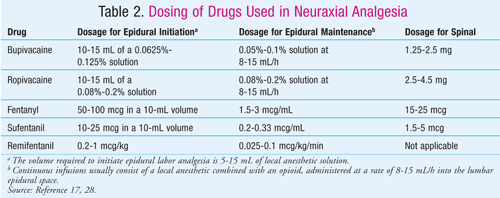
Neuraxial analgesia is currently the gold standard for pain management, and its use has increased significantly over the past 40 years.17,24 It has been observed that labor progress and outcomes are similar among women receiving either CSE or epidural analgesia.29 However, more research is required to establish whether neuraxial anesthesia is associated with increased rates of instrumental vaginal delivery.30 Side effects of neuraxial analgesia include hypotension, pruritus, maternal headache, fever, fetal bradycardia, and maternal hyperthermia.17 The patient’s ability to push may be impaired if the motor block is too dense, and there is blunting of the pressure sensation during the second stage. Neuraxial anesthesia increases the risk for operative vaginal delivery (use of forceps or a vacuum device).27
Bupivacaine is the drug of choice for spinal or dilute epidural solutions for the initiation of anesthesia as well as maintenance of labor analgesia.30 It provides favorable sensory-motor differential block at low concentrations, resulting in analgesia with motor sparing, thereby allowing ambulation.30 Bupivacaine has a long duration of action and does not produce tachyphylaxis. It is highly protein bound and therefore does not cross the placenta readily.17 Bupivacaine is effective in low concentrations for early labor and in higher concentrations as the labor progresses. For epidural or CSE analgesia, it is often combined with sufentanil or fentanyl.
Lidocaine has less differential block, a shorter duration of action, and a higher association of transient neurologic symptoms than bupivacaine. It is usually reserved for the rapid extension of an epidural.3
Ropivacaine is a newer amide local anesthetic and a homologue of bupivacaine.17 It is less cardiotoxic than bupivacaine but more so than lidocaine.31 Unlike bupivacaine, the cardiotoxicity of ropivacaine is not enhanced by progesterone. Analogous to bupivacaine, ropivacaine has favorable sensory-motor differential block at low concentrations, although when compared in equal doses, it demonstrates greater separation between sensory and motor blockade than bupivacaine.31 Ropivacaine is 60% as potent as bupivacaine when administered by the epidural route and has a similar latency and duration of action to bupivacaine.17 It is associated with significantly fewer instrumental deliveries.31
The most common opioids used in neuraxial analgesia are fentanyl and sufentanil. They both have a rapid onset of action but a short duration that can be overcome by administering a continuous epidural infusion.17
Remifentanil is an ultra short-acting mu1-opioid receptor agonist that has a rapid onset and short half-life.24 It provides modest analgesia, particularly in the first stage of labor. The amount of placental transfer is insignificant and does not seem to affect the fetus.24 Maternal side effects include mild sedation, nausea, and vomiting.24 Patients require routine oxygen saturation monitoring, with oxygen supplementation as necessary.
Neuraxial Adjuvants: Adjuvants such as epinephrine, clonidine, and neostigmine may be used to improve analgesia and decrease complications associated with a high dose of a single drug.32
Epinephrine binds to the spinal cord alpha-adrenergic receptors, decreasing uptake of local anesthetics and opioids from the epidural space as a result of vasoconstriction.17 It quickens the onset of analgesia and increases the duration of action but cannot be used for spinal analgesia. Anesthetics used in combination with epinephrine are more likely to cause motor block.
Clonidine may be similarly used in other countries, but it is not approved in the U.S. due to its sedative and hypotensive effects.32
Neostigmine has analgesic properties when administered spinally, but its use is contraindicated in patients with gastrointestinal problems.32 In an epidural combined with sufentanil or clonidine, it initiates labor analgesia without side effects and allows for a mobile epidural.
Conclusion
Collaboration between the obstetrician, neonatologist, pharmacist, and other health care providers is essential to ensure the safety of both the mother and the neonate. Misoprostol and oxytocin are the most commonly used agents for cervical ripening and labor induction. Analgesics for pain management are both systemic and neuraxial. While the need for an analgesic that provides a complete effect with no side effects particularly in the fetus is well recognized, the ideal agent is yet to be found. Pharmacists can play a vital role in drug therapy decisions and education of the patient, especially in determining pain management options during labor and delivery.
REFERENCES
1. Briggs GG, Wan SR. Drug therapy during labor and delivery, part 1. Am J Health Syst Pharm. 2006;63:1038-1047.
2. Kelsey JJ, Prevost RR. Drug therapy during labor and delivery. Am J Hosp Pharm. 1994;51:2394-2402.
3. Sweetman SC, ed. Martindale: The Complete Drug Reference. 34th ed. London, UK: Pharmaceutical Press; 2005.
4. Hofmeyr GJ, Neilson JP, Alfiervic Z, et al. Pregnancy and Childbirth: A Cochrane Pocketbook. Hoboken, NJ: Wiley; 2008.
5. Tenore JL. Methods for cervical ripening and induction of labor. Am Fam Physician. 2003;67:2123-2128.
6. Goldberg AE. Cervical ripening. Medscape Reference. http://emedicine.medscape.com/article/263311-overview#aw2aab6b5. Accessed April 4, 2012.
7. O’Brien WF. The role of prostaglandins in labor and delivery. Clin Perinatol. 1995;22:973-984.
8. Kelly AJ, Malik S, Smith L, et al. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database Syst Rev. 2009;(4):CD003101.
9. Mozurkewich EL, Chilimigras JL, Berman DR, et al. Methods of induction of labour: a systematic review. BMC Pregnancy Childbirth. 2011;11:84.
10. Harman JH Jr, Kim A. Current trends in cervical ripening and labor induction. Am Fam Physician. 1999;60:477-484.
11. Balci O, Mahmoud AS, Ozdemir S, el al. Induction of labor with vaginal misoprostol plus oxytocin versus oxytocin alone. Int J Gynaecol Obstet. 2010;110:64-67.
12. Kelly AJ, Tan B. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2001;(3):CD003246.
13. Kelly AJ, Kavanagh J, Thomas J. Relaxin for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2001;(2):CD003103.
14. McGill J, Shetty A. Mifepristone and misoprostol in the induction of labor at term. Int J Gynaecol Obstet. 2007;96:80-84.
15. Reynolds F. Labour analgesia and the baby: good news is no news. Int J Obstet Anesth. 2011;20:38-50.
16. Eltzschig HK, Lieberman ES, Camann WR. Regional anesthesia and analgesia for labor and delivery. N Engl J Med. 2003;348:319-332.
17. Wong CA. Advances in labor analgesia. Int J Womens Health. 2010;1:139-154.
18. Jones L, Othman M, Dowswell T. Pain management for women in labour: an overview of systematic reviews. Cochrane Database Syst Rev. 2012;(3):CD009234.
19. Malek A, Mattison DR. Drugs and medicines in pregnancy: the placental disposition of opioids. Curr Pharm Biotechnol. 2011;12:797-803.
20. Bucklin BA, Hawkins JL, Anderson JR, Ullrich FA. Obstetric anesthesia workforce survey: twenty-year update. Anesthesiology. 2005;103:645-653.
21. Rooks JP. Safety and risks of nitrous oxide labor analgesia: a review. J Midwifery Womens Health. 2011;56:557-565.
22. Baysinger C. Nitrous oxide for labor analgesia.
American Society of Anesthesiologists.
www.asahq.org/For-Members/Clinical-Information/Nitrous-Oxide.aspx.
Accessed August 7, 2012.
23. Anderson D. A review of systemic opioids commonly used for labor pain relief. J Midwifery Womens Health. 2011;56:222-239.
24. Hinova A, Fernando R. Systemic remifentanil for labor analgesia. Anesth Analg. 2009;109:1925-1929.
25. Cheng Y. Normal labor and delivery. Medscape Reference. http://emedicine.medscape.com/article/260036-overview#a30. Accessed April 4, 2012.
26. Ullman R, Smith LA, Burns E, et al. Parenteral opioids for maternal pain relief in labour. Cochrane Database Syst Rev. 2010;(9):CD007396.
27. Rakel D. Integrative Medicine. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012.
28. Miller RD, Eriksson LI, Fleisher LA, et al. Miller’s Anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone; 2009.
29. Norris MC, Fogel ST, Conway-Long C. Combined spinal-epidural versus epidural labor analgesia. Anesthesiology. 2001;95:913-920.
30. Satpathy HK. Labor and delivery, analgesia, regional and local. Medscape Reference. http://emedicine.medscape.com/article/149337-overview#a08. Accessed April 10, 2012.
31. Stienstra R. Clinical application of ropivacaine in obstetrics. Curr Top Med Chem. 2001;1:215-218.
32. Roelants F. The use of neuraxial adjuvant drugs (neostigmine, clonidine) in obstetrics. Curr Opin Anaesthesiol. 2006;19:233-237.
To comment on this article, contact rdavidson@uspharmacist.com.





