US Pharm. 2011;36(11):HS3-HS8.
Depression can strike anyone regardless of age, ethnic background, socioeconomic status, or gender. Major depressive disorder (MDD) is a mental disorder characterized by an all-encompassing low mood accompanied by low self-esteem and loss of interest or pleasure in normally enjoyable activities (anhedonia).1 Subtypes of major depression include psychotic, atypical, seasonal, postpartum, melancholia, and catatonic. The scope of this article focuses on the pharmacotherapy of MDD.
Prevalence
MDD is a significant health problem that affects approximately 15 million American adults yearly and about 6.7% of the U.S. population aged 18 years and older.2 Depression is the leading cause of disability in the U.S. in persons aged 15 to 44 years.3 MDD usually has an onset between the ages of 20 and 30 years and peaks between 30 and 40 years,4 with a prevalence more common in women than in men.5 According to the World Health Organization (WHO), major depression is currently the leading cause of disease burden in North America and the fourth leading cause worldwide.5
In children aged 9 to 17 years, it is estimated that the prevalence of any depressive disorder is more than 6% in a 6-month period, with 4.9% having major depression.6 Before puberty, boys and girls are equally likely to develop depressive disorders, but after age 14, females are twice as likely to have major depression or dysthymia.6
Signs and Symptoms
A person with a major depressive episode usually exhibits signs and symptoms that significantly affect a person’s personal relationships, family, work, or school life. Signs and symptoms include a depressed mood, anhedonia, weight loss/gain, insomnia/hypersomnia, fatigue/loss of energy, obsessing over thoughts, feelings of worthlessness, inappropriate guilt or regret, feelings of helplessness and hopelessness, diminished ability to think or concentrate, withdrawal from social activities, reduced sex drive, and thoughts of death or suicide.7 In severe cases, depressed people may have symptoms of psychosis that include delusions or, less commonly, hallucinations that are usually unpleasant.8 A depressed person may also report multiple somatic complaints such as fatigue, headaches, or digestive problems.7
In the elderly, depressed persons may have cognitive symptoms such as forgetfulness and a more noticeable slowing of body movements.9,10 Depression often co-exists with physical disorders such as stroke and other cardiovascular diseases, Parkinson’s disease, chronic obstructive pulmonary disease (COPD), and pain, especially if it is chronic or uncontrollable moderate-to-severe pain.11 Interestingly, depression appears to be less likely to occur, as well as quicker to remit, among individuals who have strong religious beliefs.12-14
Causes of Depression
The precise cause of depression is unknown, but it is believed to result from chemical changes in the brain due to a genetic problem triggered by stressful events, cognitive and environmental factors, or a combination of unknown causes.15,16 In depression, neural circuits in the brain responsible for the regulation of moods, thinking, sleep, appetite, and behavior fail to function properly, and critical neuro-transmitters are out of balance. Risk factors include female gender, prior episode or suicide attempts, comorbid medical or substance-related disorder, death of a close family member, lack of social support, and other stressful events.
Proposed causes include psychological, psychosocial, hereditary, evolutionary, and biological factors. The biopsychosocial model proposes that biological, psychological, and social factors all play a role in causing depression.17 Most biological theories focus on the monoamine neurotransmitters serotonin, norepinephrine, and dopamine, which are naturally present in the brain and assist communication between nerve cells.
Serotonin is hypothesized to regulate other neurotransmitter systems; decreased serotonin activity may allow these systems to act in unusual and erratic ways.18 Depression may arise when low serotonin levels promote low levels of norepinephrine.19 The monoamine hypothesis postulates that a deficiency of certain neurotransmitters is responsible for the corresponding features of depression: 1) norepinephrine may be related to alertness and energy as well as anxiety, attention, and interest in life; 2) serotonin to anxiety, obsessions, and compulsions; and 3) dopamine to attention, motivation, pleasure, and reward, as well as interest in life.20 Whatever the cause of depression, it appears to be more than just a monoamine deficiency.21 Recent studies have revealed multiple limitations of the monoamine hypothesis.22,23 A counterargument is that the mood-enhancing effect of monoamine oxidase inhibitors (MAOIs) and selective serotonin reuptake inhibitors (SSRIs) takes weeks of treatment to develop, even though the boost in available monoamine levels occurs within hours.
Diagnosis
There is no accepted clinical laboratory test for major depression. The most widely used criteria for diagnosing depressive conditions are found in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR),8 and the World Health Organization’s International Statistical Classification of Diseases and Related Health Problems (ICD-10).24,25 A clinician generally performs a comprehensive medical examination, runs clinical laboratory tests, and performs a mental status examination to determine if speech, thought patterns, or memory has been affected, and to rule out other causes of symptoms. Serum testosterone levels may be evaluated to diagnose or rule out hypogonadism, a cause of depression in men.26
Both the DSM-IV-TR and ICD-10 identify specific depressive symptoms. The ICD-10 defines three typical depressive symptoms (depressed mood, anhedonia, and reduced energy), two of which should be present to determine depressive disorder diagnosis.24,25 According to the DSM-IV-TR, there are two main depressive symptoms (depressed mood and anhedonia), at least one of which must be present to determine diagnosis of a major depressive episode (TABLE 1).8
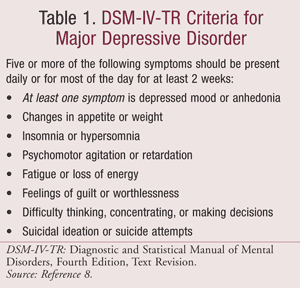
Treatment
Typically, depressed patients are treated with antidepressant medication, and in some cases, they may also receive psychotherapy or counseling. Remission is the primary goal of treatment.27,28 In this regard, all antidepressant drugs work equally well. Medications used to treat depression include SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), MAOIs, tricyclic antidepressants (TCAs), central alpha2-receptor antagonists, and norepinephrine and dopamine reuptake inhibitors (Table 2).29 Antidepressants influence the overall balance of the three neurotransmitters in the brain that regulate emotion, reactions to stress, and the physical drives of sleep, appetite, and sexuality.18 Proponents of the monoamine theory recommend choosing an antidepressant that impacts the most prominent symptoms. Anxious and irritable patients should be treated with SSRIs or SNRIs, and those experiencing a loss of energy and enjoyment of life be treated with norepinephrine- and dopamine-enhancing drugs.20
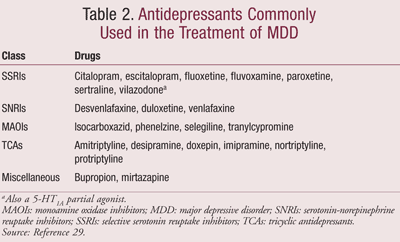
There are multiple therapeutic approaches to treating depression. These plans include the Texas Medication Algorithm Project (TMAP),30 the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trials,27 and the American Psychiatric Association (APA) Treatment Guidelines.29 All approaches utilize SSRIs, SNRIs, mirtazapine, bupropion, MAOIs, or electroconvulsive therapy (ECT) alone or a combination of adjunctive drugs such as lithium, TCAs, olanzapine, risperidone, or lamotrigine. The APA Treatment Guidelines illustrate a comprehensive approach to treating MDD and other types of depressive disorders.29 The therapy guidelines include four phases or steps for therapy: initial, continuation, maintenance, and discontinuation.
It is estimated that 30% of depressed persons seek medical treatment, and of those treated, about 30% effectively achieve remission.31 One reason for low remission rates is nonadherence to the medication regimens.31 Many patients will require several trials of therapy before achieving remission. Although clinical improvements may be seen in the first few weeks of therapy, medications must be taken regularly for 3 to 4 weeks (some 6-8 weeks) before the full therapeutic effect occurs. Hospitalization may be necessary in cases with associated self-neglect or a significant risk of harm to self or others. Treatment is usually continued for 16 to 20 weeks after remission to minimize the chance of recurrence, with up to 1 year of continuation recommended.32
Since 2007, the FDA has required that all antidepressants in the U.S. carry a black box warning on their prescribing label explaining the association between antidepressant use and increased risk for suicidality in children, adolescents, and young adults aged 18 to 24 years, especially during the first few months of treatment.33 The warning emphasizes that patients of all ages who are taking antidepressants should be closely monitored by their clinicians, especially during the initial weeks of treatment. Side effects to monitor for sudden behavioral changes include worsening of depression, withdrawal from normal social situations, agitation, irritability, anxiety, panic attacks, insomnia, aggressiveness, impulsivity, hyperactivity in actions and speech, and increased thoughts of suicide.33
Selective Serotonin Reuptake Inhibitors
SSRIs, which include fluoxetine, sertraline, paroxetine, citalopram, escitalopram, and fluvoxamine, have become the first-line treatment for major depression.29 There does not appear to be significant differences among SSRI brands in effectiveness for treating MDD.29 SSRIs work by selectively blocking the reuptake of serotonin to increase the amount of serotonin available in synapses in the brain. They are effective, have fewer and milder side effects, and are less toxic in overdose when compared to the TCAs.29 Patients who do not respond to one SSRI can be switched to another SSRI or other antidepressant.34 Another option is to switch to the atypical antidepressant bupropion.35-37 For some patients, an SNRI such as venlafaxine may be more effective than any of the SSRIs.38 Fluoxetine and escitalopram are the only antidepressants recommended for treatment of MDD in adolescents (aged 12-17 years), and fluoxetine is also approved for children aged 8 years and older.39,40 Side effects of SSRIs include dry mouth, nausea, sleepiness, dizziness, sweating, constipation, decreased appetite, and insomnia. Taking SSRIs in the morning generally avoids the insomnia issues.41
Serotonin syndrome is a very dangerous side effect and potentially fatal condition that can result from the concomitant use of two or more serotonergic drugs (e.g., SSRIs, amphetamines, dextromethorphan, lithium, linezolid, buspirone, serotonin agonists [triptans], St. John’s wort, tramadol, fentanyl). It can also occur with the use of one drug. Symptoms include confusion, marked agitation, hallucinations, elevated body temperature, diaphoresis, muscle spasms, rapid changes in blood pressure, and tachycardia.42
SSRI and 5-HT1A Partial Agonist: In January 2011, the FDA approved vilazodone hydrochloride (Viibryd) for the treatment of MDD in adults.43 Vilazodone is a combination SSRI and partial agonist of the 5-HT1A receptor. Its mechanism of action is not fully understood but is thought to be related to its enhancement of serotonergic activity in the central nervous system through selective inhibition of serotonin reuptake. The most common side effects are diarrhea, nausea, vomiting, sexual dysfunction, and insomnia. The medication should not be used with MAOIs due to risk of serious, sometimes fatal, drug interactions with serotonergic drugs.43
Serotonin-Norepinephrine Reuptake Inhibitors
SNRIs, which include venlafaxine, duloxetine, and desvenlafaxine, inhibit reuptake of serotonin and norepinephrine. They are effective in patients who do not respond to standard antidepressants or in specific patients, such as those with chronic pain (e.g., neuropathic pain, fibromyalgia, musculoskeletal pain).44-46
Side effects of SNRIs are similar to that of SSRIs.41,44-46 Patients with narrow-angle glaucoma or liver or kidney disease should not take duloxetine.45 Because duloxetine can cause liver damage, patients who drink large quantities of alcoholic beverages should not take it. Patients should immediately contact their physician if they experience any signs of liver damage, such as itching, dark urine, yellowing (jaundice) of skin and eyes, fatigue, and elevated liver function tests. Elevated blood pressure has occurred with venlafaxine and desvenlafaxine.44,46 Hypertension should be controlled before initiating treatment and blood pressure monitored regularly during treatment.
Monoamine Oxidase Inhibitors
MAOIs include phenelzine, isocarboxazid, tranylcypromine, and selegiline. They irreversibly inhibit monoamine oxidase (MAO), which results in an increase of norepinephrine and serotonin for the life of the enzyme; thus, the physiological effects of most MAOIs last up to 2 to 3 weeks.47 Although the elimination half-life of the typical MAOI is short (1.5-4 h), its physiological effects are long-lasting.48,49 MAOIs are usually prescribed only for severe depression or when other types of antidepressants do not help (i.e., treatment-resistant depression).49
Selective MAOIs have severe side effects and require restrictive dietary rules and care to avoid serious drug interactions. The interaction of tyramine with MAOIs can bring on a hypertensive crisis (a sharp increase in blood pressure) that can lead to a stroke. Studies have measured the tyramine content of food and determined that less than 6 mg per serving is generally safe.50-52 Results of these studies have led to absolute dietary restrictions for aged cheeses and meats, banana peels, broad bean (fava) pods, spoiled meats, marmite, sauerkraut, soybean products, and draft beers.51 Unlike oral MAOIs, transdermal selegiline (Emsam) does not inhibit the metabolism of dietary tyramine by MAO subtype A in the gut; therefore, it can be used without dietary restrictions at its lowest effective dose of 6 mg/24 hours.53
MAOIs interact with a number of other drugs, leading to potentially life-threatening events, and should not be taken with the following agents: amphetamines, appetite suppressants, asthma inhalers, buspirone, carbamazepine, cyclobenzaprine, decongestants, dextromethorphan, dopamine, ephedrine, epinephrine, guanethidine, levodopa, meperidine, methyl-dopa, methylphenidate, other antidepressants, reserpine, stimulants, and tryptophan.54-57 Because of their long-lasting physiological effects, there should be at least a 2-week break between taking MAOIs and starting other antidepressants.58 MAOIs can have serious interactions with some common OTC cough medications. In such cases, severe high blood pressure or dangerous reactions can occur. It is important that patients discuss any other medications they are taking or plan on taking with their physician or pharmacist.
Common side effects of MAOIs include orthostatic hypotension, drowsiness, dizziness, insomnia, and sexual dysfunction. MAOIs can also cause birth defects and should not be taken by pregnant women.49,59
Tricyclic Antidepressants
Drugs commonly listed in this group include amitriptyline, nortriptyline, imipramine, desipramine, doxepin, and protriptyline. TCAs inhibit the reuptake of serotonin and norepinephrine at the synaptic cleft. These agents were among the first antidepressants in clinical use.29,60 TCAs have a narrow therapeutic efficacy and have been associated with cardiac toxicity. They tend to cause heart rhythm disturbances for patients with certain heart diseases. Doses three to five times greater than therapeutic doses have caused toxic levels, leading to prolongation of the QT interval and eventual arrhythmias. In particular, desipramine has been associated with heart rhythm abnormalities in patients who have a family history of these problems. Care should be taken when these medications are prescribed to the elderly and to those at risk of overdose or suicide.61
Common side effects involve anticholinergic and orthostatic effects and include dry mouth, constipation, blurred vision, sexual dysfunction, weight gain, difficulty urinating (especially in patients with benign prostatic hyperplasia), drowsiness, dizziness, and orthostatic hypotension. Protriptyline can cause sun sensitivity. A number of patients discontinue their drugs due to side effects, even more so than those taking SSRIs or MAOIs.60
Miscellaneous Antidepressants
Mirtazapine: This drug enhances central noradrenergic and serotonergic activity and acts as an antagonist at central presynaptic alpha2-adrenergic inhibitory autoreceptors and heteroreceptors.62 Mirtazapine is a potent antagonist of 5-HT2, 5-HT3, and histamine-1 (H1) receptors and a moderate antagonist of peripheral alpha1-adrenergic and muscarinic receptors. Common side effects include sedation, orthostatic hypotension, impaired cognition, disinhibition, blurred vision, eye fatigue, worsening of existing eye floaters, photosensitivity, depersonalization, malaise/lassitude, increased appetite and subsequent weight gain, dry mouth, and constipation.62
Bupropion: This drug works by inhibiting the reuptake of dopamine, serotonin, and norepinephrine, an action that results in transmission of messages to other nerves.63,64 Bupropion is unique because unlike other antidepressants, its major effect is on dopamine. Bupropion causes less sexual dysfunction than SSRIs. About 25% of patients experience initial weight loss. Side effects include restlessness, agitation, sleeplessness, headache, and stomach pain. Bupropion has a risk for seizures, which increases with higher doses. High doses may also cause dangerous heart arrhythmias.63,64
Atypical Antipsychotics
If patients fail to respond to antidepressants, “augmentation” or “adjunctive treatment” may help. Atypical antipsychotics are drugs that are usually prescribed for schizophrenia or bipolar disorder, but they can also play a role in the treatment of severe depression. The only currently FDA-approved regimen for treatment-resistant depression is the olanzapine/fluoxetine combination, whereas aripiprazole and quetiapine are approved as adjunctive agents.65-67 The atypical antipsychotics are associated with a variety of relatively serious adverse effects, such as dyskinesia and neuroleptic malignant syndrome.65-67
Herbal Therapy (St. John’s Wort) for Depression
The extract from St. John’s wort (Hypericum perforatum) has been used extensively in Europe as a treatment for mild-to-moderate depression, and it now ranks among the top-selling botanical products in the U.S. Because of its increased use in America and the need to answer questions about the herb’s efficacy, the National Institutes of Health (NIH) conducted a clinical trial to determine whether a well-standardized extract of St. John’s wort was effective in the treatment of adults experiencing major depression of moderate severity. The trial found that St. John’s wort was no more effective than placebo.68
Pharmacist’s Role
The pharmacist can help maximize therapy through patient education that emphasizes that MDD is a common and treatable condition that is associated with changes in the brain’s chemistry. Patients should be encouraged to continue to take their medications regularly as directed, even if their symptoms are less noticeable or have resolved. Symptoms usually improve anywhere from 2 to 8 weeks from beginning therapy, and patients may think they no longer need the medication, or they may think it is not helping at all. Once the person is feeling better, it is important to continue the drug for an extended period of time to prevent a relapse into depression. Some agents must be stopped gradually to normalize neurotransmitter levels and prevent rebound reactions. Patients should be encouraged to never discontinue their medication or take any new prescription or nonprescription drug or herbal remedies without first talking to their health care provider.
Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber.
REFERENCES
1. Barlow DH. Abnormal Psychology: An Integrative Approach. 5th ed. Belmont, CA: Thomson Wadsworth; 2005:248-249.
2. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2005;62:617-627.
3. The World Health Organization. Annex Table 3: Burden of disease in DALYs by cause, sex, and mortality stratum in WHO regions, estimates for 2002. In: The World Health Report 2004: Changing History. Geneva, Switzerland: WHO; 2004.
4. Major depressive disorder. American Medical Network, Inc. www.health.am/psy/major-
5. Kessler RC, Berglund P, Demler O, et al; National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289:3095-3105.
6. Depression in boys and adolescent males. National Institute of Mental Health. www.nimh.nih.gov/health/
7. National Institute of Mental Health. Depression. NIH Pub. No. 11-3561. Revised 2011. www.nimh.nih.gov/health/
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Publishing; 2000.
9. Delgado PL, Schillerstrom J. Cognitive difficulties associated with depression: what are the implications for treatment? Psychiatr Times. 2009;26(3).
10. Faculty of Psychiatry of Old Age, NSW Branch, RANZCP. Consensus Guidelines for Assessment and Management of Depression in the Elderly. North Sydney, New South Wales: NSW Health Department; 2001.
11. Yohannes AM, Baldwin RC. Medical comorbidities in late-life depression. Psychiatric Times. 2008;25:52-55.
12. McCullough ME, Larson DB. Religion and depression: a review of the literature. Twin Res. 1999;2:126-136.
13. Dein S. Religion, spirituality and depression: implications for research and treatment. Prim Care Community Psychiatr. 2006;11:67-72.
14. Moreira-Almeida A, Neto FL, Koenig HG. Religiousness and mental health: a review. Rev Bras Psiquiatr. 2006;28:242-250.
15. Tsuang MT, Faraone SV. The Genetics of Mood Disorders. Baltimore, MD: Johns Hopkins University Press; 1990.
16. Lewinsohn PM, Hoberman HH, Rosenbaum M. A prospective study of risk factors for unipolar depression. J Abnorm Psychol. 1988;97:251-264.
17. U.S. Department of Health and Human Services (HHS). The Fundamentals of Mental Health and Mental Illness. Rockville, MD: HHS; 1999.
18. Biological causes of depression. All About Depression. www.allaboutdepression.com/
19. Barlow DH. Abnormal Psychology: An Integrative Approach. 5th ed. Belmont, CA: Thomson Wadsworth; 2005:226.
20. Shah N, Eisner T, Farrell M, Raeder C. An overview of SSRIs for the treatment of depression. J Pharm Soc Wisc. 1999;July/August:33-49.
21. Nutt DJ. Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatry. 2008;69(suppl E1):4-7.
22. Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894-902.
23. Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry. 2000;61(suppl 6):4-6.
24. World Health Organization (WHO) Family of International Classifications. Chapter V. Mental and behavioural disorders (F00-F99). Mood [affective] disorders (F30-F39). http://apps.who.int/
25. WHO. The ICD-10 Classification of Mental and Behavioral Disorders. Clinical Description and Diagnostic Guideline. Geneva, Switzerland: WHO; 1992.
26. Orengo C, Fullerton G, Tan R. Male depression: a review of gender concerns and testosterone therapy. Geriatrics. 2004;59:24-30.
27. Gaynes BN, Rush AJ, Trivedi MH, et al. The STAR*D study: treating depression in the real world. Cleve Clin J Med. 2008;75:57-66.
28. Depression Guideline Panel. Clinical practice guideline, number 5. Depression in Primary Care: Volume 1. Detection and Diagnosis. AHCPR Pub. No. 93-0551. Rockville, MD: HHS, Agency for Health Care Policy and Research; 1993.
29. American Psychiatric Association. Practice Guideline For The Treatment of Patients With Major Depressive Disorder, Third Edition. November 2010. www.psychiatryonline.com/
30. Miller AL, Hall CS, Buchanan RW, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2003 update. J Clin Psychiatry. 2004;65:500-508.
31. Nemeroff CB. Improving antidepressant adherence. J Clin Psychiatry. 2003;64:25-30.
32. Thase ME. Preventing relapse and recurrence of depression: a brief review of therapeutic options. CNS Spectr. 2006;11(suppl 15):12-21.
33. Antidepressant use in children, adolescents, and adults. FDA. May 2, 2007. www.fda.gov/Drugs/DrugSafety/
34. Whooley MA, Simon GE. Managing depression in medical outpatients. N Engl J Med. 2000;343:1942-1950.
35. Zisook S, Rush AJ, Haight BR, et al. Use of bupropion in combination with serotonin reuptake inhibitors. Biol Psychiatry. 2006;59:203-210.
36. Rush AJ, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354:1231-1242.
37. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243-1252.
38. Papakostas GI, Thase ME, Fava M, et al. Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? A meta-analysis of studies of newer agents. Biol Psychiatry. 2007;62:1217-1227.
39. National Institute for Health and Clinical Excellence. Depression in Children and Young People: Identification and Management in Primary, Community and Secondary Care. Leicester, UK: British Psychological Society; 2005.
40. Lexapro (escitalopram oxalate) package insert. St. Louis, MO: Forest Laboratories; May 2011.
41. Mayers AG, Baldwin DS. Antidepressants and their effect on sleep. Hum Psychopharmacol. 2005;20:533-559.
42. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112-1120.
43. Viibryd (vilazodone) package insert. St. Louis, MO: Forest Laboratories; March 2011.
44. Effexor XR (venlafaxine) package insert. Philadelphia, PA: Wyeth Pharmaceuticals Inc; July 2009.
45. Cymbalta (duloxetine) package insert. Indianapolis, IN: Eli Lilly and Company; September 2011.
46. Pristiq (desvenlafaxine) package insert. Philadelphia, PA: Wyeth Pharmaceuticals Inc; July 2011.
47. Cooper AJ. Tyramine and irreversible monoamine oxidase inhibitors in clinical practice. Br J Psychiatry Suppl. 1989;(6):38-45.
48. Mallinger AG, Smith E. Pharmacokinetics of monoamine oxidase inhibitors. Psychopharmacol Bull. 1991;27:493-502.
49. Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10:239-248.
50. Horwitz D, Lovenberg W, Engelman K, Sjoerdsma A. Monoamine oxidase inhibitors, tyramine, and cheese. JAMA. 1964;188:1108-1110.
51. Gardner DM, Shulman KI, Walker SE, Tailor SA. The making of a user friendly MAOI diet. J Clin Psychiatry. 1996;57:99-104.
52. Shulman KI, Walker SE. Refining the MAOI diet: tyramine content of pizza and soy products. J Clin Psychiatry. 1999;60:191-193.
53. Patkar AA, Pae CU, Masand PS. Transdermal selegiline: the new generation of monoamine oxidase inhibitors. CNS Spectr. 2006;11:363-375.54. Lorenz RA, Vandenberg AM, Canepa EA. Serotonergic antidepressants and linezolid: a retrospective chart review and presentation of cases. Int J Psychiatry Med. 2008;38:81-90.
55. Miller DG, Lovell EO. Antibiotic-induced serotonin syndrome. J Emerg Med. 2011;40:20-27.
56. Das PK, Warkentin DI, Hewko R, Forrest DL. Serotonin syndrome after concomitant treatment with linezolid and meperidine. Clin Infect Dis. 2008;46:264-265.
57. Packer S, Berman SA. Serotonin syndrome precipitated by the monoamine oxidase inhibitor linezolid. Am J Psychiatry. 2007;164:346-347.
58. Marangell LB. Switching antidepressants for treatment-resistant major depression. J Clin Psychiatry. 2001;62(suppl 18):12-17.
59. Evans DL, Davidson J, Raft D. Early and late side effects of phenelzine. J Clin Psychopharmacol. 1982;2:208-210.
60. Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacology. 2007;151:737-748.
61. Teicher MH, Glod CA, Cole JO. Antidepressant drugs and the emergence of suicidal tendencies. Drug Saf. 1993;8:186-212.
62. Gorman JM. Mirtazapine: clinical overview. J Clin Psychiatry. 1999;60(suppl 17):9-13.
63. Terry P, Katz JL. Dopaminergic mediation of the discriminative stimulus effects of bupropion. Psychopharmacology. 1997;134:201-212.
64. Learned-Coughlin SM, Bergstrom M, Savitcheva I, et al. In vivo activity of bupropion at the human dopamine transporter. Biol Psychiatry. 2003;54:800-805.
65. Symbyax (olanzapine and fluoxetine hydrochloride) package insert. Indianapolis, IN: Eli Lilly & Co; August 2011.
66. Seroquel XR (quetiapine fumarate) package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; July 2011.
67. Abilify (aripiprazole) package insert. Tokyo, Japan: Otsuka Pharmaceutical Company; February 2011.
68. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St. John’s wort) in major depressive disorder: a randomized, controlled trial. JAMA. 2002;287:1807-1814.
To comment on this article, contact rdavidson@uspharmacist.com.





