US Pharm. 2015;40(Specialty&Oncology suppl):2-5.
ABSTRACT: The incidence of pregnancy-associated breast cancer is expected to increase as more women postpone childbearing to later in life. There is limited data on treating pregnant women with breast cancer with chemotherapy and even less data on the outcomes of children exposed to chemotherapy in utero. Treatment guidelines remain the same for pregnant women with breast cancer as for nonpregnant women, with some modifications to protect the fetus. Surgery has been shown to be safe in all stages of pregnancy, whereas chemotherapy should be delayed until after the first trimester, and radiation should be delayed until after delivery. Termination of the pregnancy does not affect maternal prognosis.
Breast cancer is the most common malignancy in pregnancy and occurs in approximately 1 in 3,000 women.1 Breast cancer during pregnancy is rare; however, as more women are having children later in life, the risk of developing breast cancer during pregnancy is increasing. Gestational or pregnancy-associated breast cancer (PABC) can be defined as being diagnosed with breast cancer during pregnancy or 1 year postpartum and is the most frequently diagnosed cancer during pregnancy.2
The treatment goal for PABC is the same as for a nonpregnant woman with breast cancer: to control the disease. However, considerations in traditional treatment must now be made to protect the fetus while maximizing benefits to the patient. Traditional treatment also poses unique challenges as there are limited data on the safety of using chemotherapy and radiation during pregnancy. Most of the clinical evidence is limited to case reports and retrospective cases.
Diagnosis
Delays in the diagnosis of PABC are common, as long as 5 to 15 months from the onset of symptoms due to the fact that hormone changes during pregnancy make a woman’s breasts larger, more tender, and denser, making it difficult to detect an abnormality.2,3 A woman with PABC is also at an increased risk of having a more advanced form of breast cancer than a nonpregnant woman because physicians and patients may mistakenly attribute findings consistent with breast cancer to normal pregnancy-induced changes.3
When abnormalities are found in the breast of a pregnant woman, mammograms and ultrasounds may be used as diagnostic tools. Mammography poses low risk of radiation to the developing fetus if proper shielding is used.4 However, ultrasonography has a higher sensitivity than mammography in detecting a breast mass and is a more accurate and safer diagnostic tool.5 Diagnosis may also be more safely accomplished using fine-needle aspiration, core biopsy, or excisional biopsy under local anesthesia.1 Core or excisional biopsy is considered the gold standard for the diagnosis of breast cancer.6 It is important for the pathologist to be informed that the woman is pregnant to avoid false-positive results due to misinterpretation of the pregnancy-induced changes of the breast.
Therapeutic Management
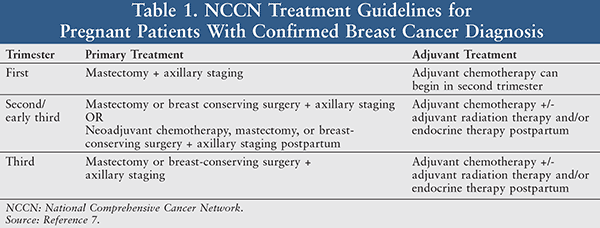
Treatment for PABC should adhere to the same criteria as nonpregnant women with breast cancer, with considerations to protect the developing fetus. Treatment options include surgery, chemotherapy, and radiation. There has been no evidence to suggest that termination of the pregnancy will improve prognosis, and it is not routinely indicated.6 According to the most recent version of the National Comprehensive Cancer Network (NCCN) guidelines for breast cancer during pregnancy (TABLE 1), chemo-therapy should not be administered during the first trimester of pregnancy, and radiation therapy should not be administered during any trimester of pregnancy.7
Radiation: Local breast radiation therapy necessary to complete breast-conserving treatment or postmastectomy radiation is contraindicated in pregnancy due to the risks associated with fetal exposure to radiation. Radiotherapy could cause miscarriage, teratogenicity, abnormal growth, mental retardation, and malignant disorders in the fetus or child.6 Therefore, in PABC, radiation therapy should be reserved until after delivery.
Surgery: Surgical procedures can be safely performed throughout all trimesters of pregnancy with minimal risk to the developing fetus or continuation of the pregnancy.8 With modern surgical and anesthetic techniques, maternal and fetal risk is low, as there has not been a significant increase in the risk of maternal death, birth defects, or late neuro-developmental delays.3 Mastectomy with axillary lymph node dissection is often the preferred breast-conserving surgery option due to the risks of fetal radiation exposure.8 An advantage of having a mastectomy is that it may limit the need for radiation. Breast reconstruction should be delayed until after delivery.
Pharmacologic Treatment
Chemotherapy: All chemotherapy medications are capable of crossing the placenta; however, chemotherapy is considered to be safe when used during the second and third trimesters of pregnancy, as demonstrated in two series of women reported from the MD Anderson Cancer Center and The Royal Marsden Hospital.6 Cytotoxic chemotherapy has a high potential for teratogenicity during organogenesis (weeks 2 through 8) and spontaneous abortion.9 Therefore, the use of chemotherapy is contraindicated in the first trimester of pregnancy. Chemotherapy exposure can still, however, result in complications to the baby such as preterm delivery, low birth-weight, transient tachypnea of the newborn, and intrauterine growth restriction. The decision to use chemotherapy during pregnancy must be weighed against the effect of treatment delay on maternal survival versus the risks to the fetus.
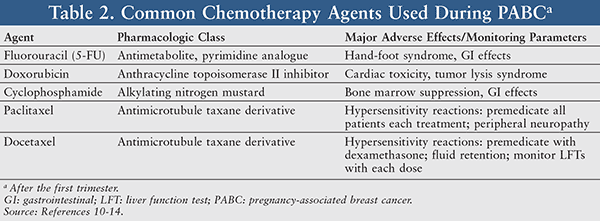
Common therapeutic agents that have been used in PABC are fluorouracil (5-FU), doxorubicin, cyclophosphamide, and the taxane derivatives paclitaxel and docetaxel (TABLE 2).10-14 Most studies evaluating chemotherapy in PABC have observed doxorubicin and cyclophosphamide (AC) and fluorouracil, doxorubicin, and cyclophosphamide (FAC), but few studies have observed the safety of taxanes, which play a pivotal role in adjuvant regimens in nonpregnant patients.8,9 To date, the use of taxanes in PABC has primarily been documented through case reports. In a study that looked at contemporary multidisciplinary treatment of PABC in an academic setting focusing on maternal and fetal outcomes, 8.3% of patients in the cohort who received AC also received 12 weekly cycles of paclitaxel during pregnancy without evident undesirable effects.15 More studies are needed to confirm the safety of giving taxanes in pregnancy.
The dosages of chemotherapy during pregnancy are calculated the same way as for nonpregnant patients, using the body surface area (BSA). The dose of chemotherapy should be calculated on the basis of the actual body weight of the pregnant woman and adapted as the weight adjusts throughout the pregnancy.16 Chemotherapy dosages in general should be calculated using the actual body weight of the patient and adjusted for obesity or being underweight.
FAC is an IV chemotherapy regimen used during breast cancer.10-12 The cycle is repeated every 21 days for up to 9 cycles. The dose of fluorouracil is 500 mg/m2/day on days 1 and 8; doxorubicin is 50 mg/m2 on day 1, and cyclophosphamide is 500 mg/m2 on day 1. A CBC with differential needs to be done at baseline and before each cycle of chemotherapy, as the dosages may be altered or held based on the patient’s white blood cell (WBC) count, platelets, or absolute neutrophil count (ANC). Liver function tests or LFTs (aspartate aminotransferase [ALT], alanine aminotransferase [AST], bilirubin, and alkaline phosphatase), and serum creatinine should also be monitored at baseline and prior to each cycle, as some of these drugs are metabolized via the hepatic or renal pathway, or both, and need to be adjusted or withheld according to the patient’s levels.10-12
5-FU is an antimetabolite pyrimidine analogue that interferes with DNA and RNA synthesis.10 After activation, 5-fluoroxyuridine monophosphate (F-UMP), an active metabolite, is incorporated into RNA to replace uracil and inhibit cell growth; the active metabolite F-UMP inhibits thymidylate synthetase, depleting thymidine triphosphate, which is a necessary component of DNA synthesis. Adverse effects may include, but are not limited to, hand-foot syndrome, vomiting, diarrhea, alopecia, leukopenia, and thrombocytopenia. 5-FU undergoes hepatic metabolism and should be used with caution in patients with extreme hepatic impairment.10
Doxorubicin is an anthracycline topoisomerase II inhibitor. It inhibits DNA and RNA synthesis through intercalation between DNA base pairs by inhibiting topoisomerase II and by steric obstruction, and intercalates at points of local uncoiling of the double helix.11 Cardiac toxicity is a major adverse effect of doxorubicin, manifested in the most severe form as congestive heart failure (CHF), which can occur during therapy or months to years after the discontinuation of doxorubicin. As the dose cumulates (>400 mg/m2), there is a rapid increase in the development of CHF, although cardiac toxicity can still occur at low doses regardless of cardiac risk factors. A multigated acquisition (MUGA) scan or echocardiogram is recommended prior to the start of therapy, to be repeated every 3 months while on treatment and at follow-up after doxorubicin has been discontinued. Other adverse effects of doxorubicin may include alopecia, severe nausea/vomiting, discoloration of urine, tumor lysis syndrome, leukopenia, neutropenia, anemia, and thrombocytopenia. The dosage of doxorubicin may need to be adjusted if hepatic impairment is present.11
Cyclophosphamide belongs to the class of alkylating nitrogen mustards. It prevents cell division by cross-linking DNA strands and decreasing DNA synthesis, and is cell-cycle phase nonspecific.12 Cyclophosphamide is a prodrug that must be metabolized to active metabolites in the liver; therefore, in patients with severe hepatic impairment, use should be avoided. There are no recommendations per the manufacturer; however, caution should be used in patients with renal and/or hepatic dysfunction and the dosage adjusted accordingly. Common adverse effects may include alopecia, nausea, vomiting, diarrhea, neutropenic fever, thrombocytopenia, leukopenia, anemia, and myelosuppression.12
AC plus paclitaxel is another common IV breast cancer regimen used in nonpregnant patients.11,12 This regimen has been used successfully in PABC patients as well; however, there have not been many studies focusing on the effects of taxanes during and after pregnancy. AC is given every 21 days for a total of 4 cycles, followed by 12 weekly cycles of paclitaxel. The dose of doxorubicin is 60 mg/m2 and the dose of cyclophosphamide is 600 mg/m2. After four cycles, paclitaxel is then administered at a dose of 80 mg/m2 once weekly for 12 cycles.11,12
Alternatively, AC can also be given as a “dose-dense” treatment every 2 weeks versus every 3 with the granulocyte colony-stimulating growth factor (G-CSF) pegfilgrastim or filgrastim.9,15 The growth factors stimulate the production, activation, and maturation of the neutrophils. Pegfilgrastim has a prolonged duration of effect relative to filgrastim and a reduced renal clearance.17
Taxanes: Paclitaxel is an antimicrotubule taxane derivative. It promotes microtubule assembly by enhancing the action of tubulin dimers, stabilizing existing microtubules and inhibiting their disassembly, interfering with the late G2 mitotic phase, and inhibiting cell replication.13 Paclitaxel can also distort mitotic spindles, resulting in the breakage of chromosomes, and may suppress cell proliferation and modulate immune response. Hypersensitivity reactions can occur with paclitaxel, such as dyspnea, hypotension, hives, and angioedema, as well as severe anaphylaxis; therefore, all patients must be pretreated each time with a corticosteroid, diphenhydramine, and a histamine-2 (H2) antagonist. Patients who react to paclitaxel with a severe hypersensitivity should not be rechallenged with paclitaxel. Some common adverse effects of paclitaxel include alopecia, peripheral neuropathy, nausea/vomiting, hematologic effects such as neutropenia, and myalgia. Dosage adjustments may be needed for hepatic impairment of elevated LFTs.13
Docetaxel is a microtubule taxane derivative that has been used in the treatment of breast cancer and in some cases of PABC.14 It also follows the regimen of AC—every 21 days for 4 cycles—but is dosed at 100 mg/m2 every 21 days for 4 cycles. Docetaxel, like paclitaxel, can cause severe hypersensitivity reactions. A corticosteroid, such as dexamethasone, is given for 3 days starting the day prior to treatment to reduce the chance of a hypersensitivity reaction and also to prevent fluid retention. Dexamethasone does cross the placenta and is indicated for use only after the first trimester. The lowest effective dose possible should be used for the shortest amount of time since there are data that have shown dexamethasone to affect fetal growth, such as by causing low birth-weight. In patients with hepatic impairment, the dose of docetaxel needs to be reduced or withheld based on the LFTs. LFTs need to be monitored with each dose of docetaxel.14
Some data suggest that infants of PABC mothers treated with chemotherapy might have earlier delivery dates; therefore, chemotherapy should not be given after 35 weeks’ gestation in order to minimize the risk of neutropenia at delivery.18 Delivery should be delayed for 3 weeks after discontinuation of chemotherapy to allow for placental drug excretion from the fetus to avoid marked bone marrow and systemic toxicity in the baby.19 If oncological treatment is still needed after delivery, it may be continued immediately following a vaginal delivery and 1 week after an uncomplicated caesarean section.18 Breastfeeding is contraindicated during chemotherapy, as data on effects during lactation are limited. It is unclear how much toxicity can be attributed to chemotherapy agents during lactation, as different agents can vary in their concentration in the breast milk.
Supportive Medications: Antiemetics such as promethazine, selective serotonin (5-HT3 ) antagonists (granisetron, ondansetron, palonosetron), and dexamethasone have been used to treat severe nausea and vomiting associated with chemotherapy in pregnant women. Ondansetron is a Pregnancy Category B drug and is reported to have the longest safety record in pregnancy.6 Long-term use of dexamethasone should be avoided due to potential maternal and fetal risks as it crosses the placenta.
G-CSFs such as pegfilgrastim and filgrastim have also been reported to be safe during pregnancy.20 These agents are given during chemotherapy to provide hematologic support and to reduce the risk of maternal sepsis from neutropenic adverse events.6
Hormone Therapy: Hormonal therapy is often used as adjuvant treatment after surgery or for advanced breast disease. Tamoxifen, a selective estrogen receptor modulator, has both agonist and antagonist activity. It is used in premenopausal women with estrogen receptor-positive breast cancers and for palliative treatment of advanced metastatic disease. Tamoxifen has been linked to high rates of birth defects such as craniofacial malformations, ambiguous genitalia, and fetal death and is, therefore, not recommended during pregnancy.16
Targeted Therapy: Trastuzumab, pertuzumab, ado-trastuzumab emtansine, and lapatinib all target human epidermal growth factor receptor 2 (HER2)–positive breast tumors and play an important role in the treatment of HER2-positive breast cancer in nonpregnant women. Unfortunately, none of these medications is considered to be safe for the fetus and therefore cannot be used as a treatment regimen for PABC.16
Methotrexate: Methotrexate should not be used as a potential treatment in PABC. It is contraindicated in any stage of pregnancy because of its teratogenic potential and abortifacient effects.20
Ethical Concerns
PABC can raise complex ethical concerns for both the patient and the healthcare team. There is no medical evidence to suggest that termination of the pregnancy will improve prognosis of the mother.6 The decision to end or proceed with the pregnancy during PABC is a personal decision that ultimately must be made by the mother after extensive discussions with the medical team. The impact of the diagnosis of PABC on maternal health, fetal outcome, and risks to the fetus should be considered on an individual basis.
REFERENCES
1. National Cancer Institute. Breast Cancer and Pregnancy (PDQ). Bethesda, MD: National Cancer Institute; March 2014. www.cancer.gov/cancertopics/pdq/treatment/breast-cancer-and-pregnancy/HealthProfessional. Accessed August 1, 2014.
2. American Cancer Society. Breast cancer during pregnancy. www.cancer.org/cancer/breastcancer/moreinformation/pregnancy-and-breast-cancer. Accessed August 1, 2014.
3. Pereg D, Koren G, Lishner M. Cancer in pregnancy: gaps, challenges and solutions. Cancer Treat Rev. 2008;34:302-312.
4. Gelber S, Coates AS, Goldhirsh A, et al. Effect of pregnancy on overall survival after the diagnosis of early-stage breast cancer. J Clin Oncol. 2001;19(6): 1671-1675.
5. Yang WT, Dryden MJ, Gwyn K, et al. Imaging of breast cancer diagnosed and treated with chemotherapy during pregnancy. Radiology. 2006;239:52-60.
6. Padmagirison R, Gajjar K, Spencer C. Management of breast cancer during pregnancy. Obstetrician & Gynaecologist. 2010;12:186-192.
7. National Comprehensive Cancer Network. NCCN Guidelines Version 3.2014. Breast Cancer During Pregnancy. Fort Washington, PA: NCCN; 2014.
8. Gwyn KM, Theriault RL. Breast cancer during pregnancy. Curr Treat Options Oncol. 2000;1:239-243.
9. Molckovsky A, Madarnas Y. Breast cancer in pregnancy: a literature review. Breast Cancer Res Treat. 2008;108:333-338.
10. Fluorouracil. LexiComp Online. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1797299#pha. Accessed August 1, 2014.
11. Doxorubicin. LexiComp Online. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6790#pha. Accessed August 1, 2014.
12. Cyclophosphamide. LexiComp Online. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6674#pha. Accessed August 1, 2014.
13. Paclitaxel. LexiComp Online. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7427#aot. Accessed August 1, 2014.
14. Docetaxel. LexiComp Online. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6775. Accessed August 1, 2014.
15. Meisel JL, Economy KE, Cavillo KZ, et al. Contemporary multidisciplinary treatment of pregnancy-associated breast cancer. SpringerPlus. 2013;2:297.
16. Amant F, Loibl S, Neven P, Van Calsteren K. Breast cancer in pregnancy. Lancet. 2012;379:570-579.
17. Pegfilgrastim. LexiComp Online. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7445. Accessed January 12, 2015.
18. Loibl S, von Minckwitz G, Gwyn K, et al. Breast cancer during pregnancy. International recommendations from an expert meeting. Cancer. 2006;106(2):237-246.
19. Pavlidis N, Pentheroudakis G. The pregnant mother with breast cancer: diagnostic and therapeutic management. Cancer Treat Rev. 2005;31:439-447.
20. Litton J, Theriault R. Gestational breast cancer: treatment. UptoDate. www.uptodate.com. Accessed August 1, 2014.
To comment on this article, contact rdavidson@uspharmacist.com.





