US Pharm.
2006;11:HS-30-HS-37.
Despite
the gaps in knowledge regarding the safety and efficacy of psychotropic
medications in children and adolescents, health care providers continue to
prescribe these agents to treat mental disorders, including major depression,
bipolar disorder, attention-deficit/hyperactivity disorder (ADHD), autism
spectrum disorders, substance abuse and dependence, and eating disorders. Many
of these disorders were once considered problems experienced in adulthood
only, but an estimated one in 10 children and adolescents in the United States
suffers from mental illness that is severe enough to lead to personal, social,
and/or academic impairment. Recently, these illnesses have been better
documented and fully recognized in childhood and adolescence. An increase in
prescribing rates of stimulants, antidepressants, clonidine, and
antipsychotics in preschoolers has been observed.1 Of note, most of
these agents are not FDA-approved for use in the pediatric population or have
limited indications in school-age children and/or adolescents.
Diagnosing a mental disorder
in children, especially preschool children, can be difficult. Comorbidities
are common and may complicate management, requiring polypharmacy, although
many of the illnesses can be transient or may be adequately managed with
cognitive behavioral therapy (CBT) alone to improve deficits in daily
functioning.2 Some symptoms can be treated with psychotropic
medications (e.g., antidepressants, anxiolytics, hypnotics, antipsychotics,
neuroleptics, and stimulants) as adjunct therapy. However, these medications
should be recommended judiciously, particularly if it is an off-label
indication, at the lowest possible doses, to reduce side-effect potential.
To complicate matters further, children are
in a constant state of rapid change physically, cognitively, and emotionally,
throughout their developmental years. Physicians should heed this fact when
selecting psychotropic medication(s), as it may affect adherence to the
regimen. Often, the dosage and indication for psychotropic medications are
based on extrapolated adult data, which are not always appropriate for very
young children, school-age prepubertal children, or adolescents. Some
toxicities in adults have yet to be discovered in children. Furthermore,
long-term consequences from administration of psychotropic medications at a
young age, especially on the brain, are unknown. The bottom line is that more
epidemiologic and clinical research is needed.1,3 The FDA's
Modernization Act and Best Pharmaceuticals for Children Act provide incentives
for the pharmaceutical industry to conduct studies in the pediatric population.
3 However, the growing concern about the safety of these psychotropic
medications in children and adolescents is offset by evidence indicating that
treatment benefits outweigh the risks of the medications.4 Clinical
pharmacy has become more important than ever, with the growing need to learn
more about children who are treated for all ranges of mental illness. (See
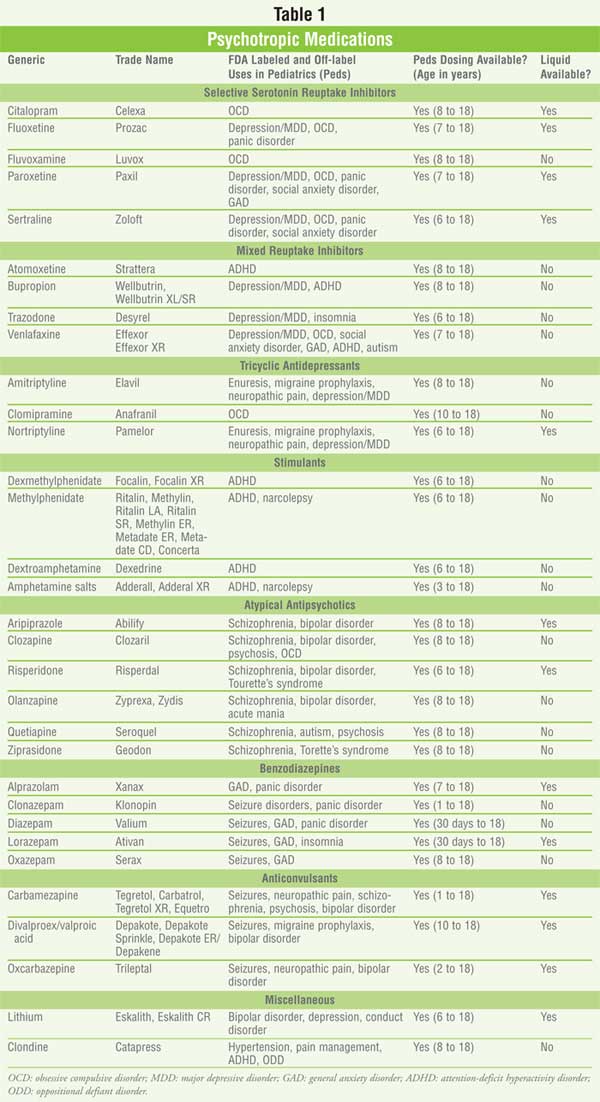
Table 1)
Antidepressants
Major Depressive Disorder:
Childhood depression is among the most prevalent of pediatric mood disorders
and is a leading cause of morbidity and mortality in children. Although major
depressive disorder (MDD), the most severe form of depression, has not been
consistently linked to suicide, it remains an important contributor to
suicidal behavior and suicide.5,6 Since 2000, approximately 2% of
children and 4% to 8% of adolescents with MDD have died due to self-harm, the
third leading cause of death among children.7-9 Nonetheless,
suicide secondary to depression is preventable.
Combining therapy with CBT is
a crucial component of complete and balanced MDD management. Fluoxetine, a
selective serotonin reuptake inhibitor (SSRI), is recommended as first-line
therapy initially at 10 to 20 mg every 24 hours for children ages 8 to 18
years. Dose titration to the lowest effective dose (range, 10 to 60 mg/day) in
increments of 10 mg should be done after four to six weeks of observation and
follow-up postinitiation.10 If fluoxetine is not effective, other
SSRIs (e.g., sertraline, citalopram, escitalopram, fluvoxamine, paroxetine)
and mixed reuptake inhibitors (e.g., venlafaxine, mirtazapine, bupropion)
should be considered as second- and third-line options, respectively. Other
reasons for switching medications include intolerance, comorbid diseases,
potential drug interactions, and drug formulation. Close monitoring for
suicidal ideation, at least during the first month following drug initiation,
is strongly recommended. Notably, limited data exist on the safety and
efficacy of antidepressants and mood stabilizers in school-age prepubertal
children.
Eating Disorders:
Eating disorders usually develop during adolescence but can also occur in
early childhood. They can coexist with depression, substance abuse, and
anxiety disorders. Females are more likely to suffer from an eating disorder
than are males. About 0.5% to 3.7% of females suffer from anorexia nervosa,
and 1.1% to 4.2% from bulimia nervosa. Nonpharmacologic treatment is
recommended to restore weight lost. Established weight gain is followed by
SSRI treatment for weight maintenance and resolution of mood and anxiety
symptoms. Fluoxetine is the only SSRI approved for reducing symptoms of
bulimia nervosa.11
Side Effects of
Antidepressants:
Notable side effects with antidepressants include sedation, especially the
tricyclic antidepressants (TCAs) trazadone, mirtazapine, and nefazodone. While
most SSRIs cause insomnia, paroxetine may induce mild sedation. SSRIs may also
cause declines in daytime and driving performance and increase potential for
involvement in motor vehicle accidents.12 Although akathisia is a
common result of antipsychotic use, SSRIs can also produce akathisia. Serum
electrolytes should be checked if patients taking SSRIs present with
unexplained mental slowing, somnolence, reduced food intake, vomiting, or
seizures.13 TCAs can cause weight gain.14 Furthermore,
compliance may be affected by their unpleasant taste, ability to alter taste
modalites, and effect of xerostomia.15
SSRIs have recently gained a
great deal of attention after data from the landmark trial Treatment for
Adolescents with Depression Study, were published in 2004.16
Despite significant improvements in Clinical Global Impression in groups
treated with fluoxetine and decreases in the overall suicidal ideation by the
end of the study, the elevated risk for harm-related adverse events, both
suicide related or otherwise, was alarming. None of the odds ratios for
suicide-related events was statistically significant. In September 2004, a
causal link was established between the newer antidepressants used for MDDs.
Subsequently in October 2004, the FDA mandated changes in antidepressant
advertisements, package inserts, and information sheets to include a black box
warning about the increased risk of suicidality.17
Mood Stabilizers
Pediatric bipolar disorder can
result in impaired family and peer relationships, poor academic performance,
higher levels of substance abuse, increased rates of suicide attempts and
completion, legal difficulties, and multiple hospitalizations. In fact, 30% to
40% of pediatric psychiatric inpatients have bipolar disorder. Children and
adolescents with bipolar disorder often present with severe mood swings,
disruptive behaviors, short sleep periods, intrusiveness, and hypersexuality.
18 They are also more prone to violence.19 Cycles of manic
symptoms, major depression, and cyclothymia can occur.18 Of note,
antidepressants and corticosteroids can result in a mimicry of mood disorders
and should be included in the diagnostic differential.18
Comorbidities such as ADHD and conduct disorder (CD) are common in children
and adolescents.
Lithium:
Lithium is FDA approved for the treatment of acute mania and bipolar disorder
in adolescents or children (ages 12 to 18), especially if the patient presents
with classic euphoric mania without psychotic symptoms.18 It has
the longest history of use in children and adolescents and should be
considered first.19 Dosage should be titrated to 30 mg/kg/day in
two to three divided doses, for a target serum concentration of 0.8 to 1.2
mEq/L. Common side effects are hypothyroidism, nausea, polyuria, polydipsia,
tremor, acne, and weight gain. The latter two side effects may be not be well
tolerated by adolescents; thus, compliance may become an issue. Monitoring of
serum lithium levels and renal and thyroid function are recommended at
baseline and every six months.13,18,19 Electrocardiography may also
be useful.19
Anticonvulsants:
Sodium divalproex is
widely used in children and adolescents with bipolar depression and aggressive
behavior, but few controlled trials have been conducted.18,19 The
dose should be initiated at 20 mg/kg/day, which produces a serum level of 80
to 120 mcg/mL. Common side effects include weight gain, nausea, sedation, and
tremor. Until the risk of polycystic ovary disease is clearly defined, weight
or menstrual abnormalities, hirsutism, and acne should be monitored.18
Valproic acid can also have a mild antithyroid effect.13 Monitoring
of the complete blood count (CBC), liver function tests (LFTs), thyroid
function tests, weight, and serum valproic levels is advised. Females should
also avoid pregnancy, since the drug is teratogenic.19
Carbamazepine is used as
monotherapy or adjunctive therapy for bipolar disorder and violent behavior.
The dose should be titrated slowly, and frequent monitoring of serum
concentrations is necessary if patients are concurrently taking other
medications that affect the CYP-450 system. It also is a CYP-450 enzyme
inducer. Side effects include aplastic anemia, severe dermatologic reactions
(e.g., Stevens-Johnson syndrome), hyponatremia, nausea, and sedation.13,18
Monitoring of CBC, LFTs, serum electrolytes, and serum carbamazepine level
(for toxicity) is recommended. Females taking oral contraceptives should be
counseled about using an additional mode of contraception or abstinence.
Weight gain is also a concern for 25% of patients.19
Oxcarbazepine is an analog to
carbamazepine and appears to be promising in adults with mania.20
In children ages 7 to 18 years with bipolar I disorder, it is no better than
placebo,21 but anecdotally it is an effective treatment for
aggression.19 As with carbamazepine, hyponatremia can occur,
although less frequently in children, and monitoring is recommended.13,18
Females should be counseled about effective contraception.
Antipsychotics:
Atypical antipsychotics provide a broader spectrum of efficacy with a better
safety profile than the older antipsychotics and are preferred in practice.
The atypical agents block dopamine2 neurotransmission and increase
serotonin levels. They are prescribed to treat not only schizophrenia but also
acute bipolar mania, bipolar depression, treatment-resistant depression, and
posttraumatic stress disorder due to their mood-stabilizing properties.18
None has been approved for use in the pediatric population, but clozapine,
risperidone, olanzapine, and quetiapine have been studied for mania and may be
effective, especially if psychotic symptoms are also present.2,18
They appear to be clinically more efficacious than traditional mood
stabilizers.2
Risk of weight gain with
atypical antipsychotics appears to be higher in children.13
Significant weight gain can increase morbidity secondary to metabolic syndrome
involving type 2 diabetes, dyslipidemia, hypertension, and cardiovascular
disease.13,22 A personal and family history, along with baseline
measurements of waist circumference, blood pressure, fasting blood glucose,
and fasting lipid profile, should be obtained prior to initiating
antipsychotics, with reassessments at four, eight, and 12 weeks after
initiating or changing therapy. Further evaluations should be performed every
three months.14,18,23 The propensity for weight gain and
development of the metabolic syndrome is highest with clozapine and
olanzapine, followed by risperidone, quetia pine, ziprasidone, and
aripiprazole.13
Akathisia can also occur with
high doses, rapid increments of dosage, and higher drug potency. Neuroleptic
malignant syndrome has been reported with atypical antipsychotics as well.
Patients who are agitated, dehydrated, malnourished, taking large doses of
higher-potency antipsychotics, or undergoing rapid increments of dosage should
be monitored closely.13 Longer-term use resulted in mild to
moderate tremor, muscle rigidity, and restlessness in a small number of
patients.21 Hyperprolactinemia is more pronounced in postpubertal
children and adolescents than in adults.13 Monitoring for
amenorrhea or oligomenorrhea, breast enlargement or engorgement, galactorrhea,
decreased libido, erectile dysfunction, osteoporosis, and hirsutism is
recommended.13 Serum prolactin levels should be checked only if the
patient is symptomatic, and dosage of the current antipsychotic should be
reduced or the patient should be switched to a different drug if prolactin
levels are above normal but less than 200 ng/mL. Levels above 200 ng/mL
require further medical work-up. In some patients, symptoms may resolve within
six to 12 months of continued treatment.13
Autism and Other
Pervasive Developmental Disorders:
Autism affects one in 500 children.24 Symptoms of autism, such as
stereotypies, compulsions, aggression, extreme intolerance of change, and
self-injurious behavior, can be treated with antipsychotics.3,22
However, the safety and efficacy of this practice have not been determined.
3 The onset of autism, other pervasive disorders, and ADHD typically
occurs during preschool years. Risperidone is the best-studied agent in
treating symptoms of autism spectrum disorder. A recent six-month trial of
risperidone versus placebo in 5- to 17-year-olds demonstrated that
risperidone, at mean doses of 1.5 to 1.8 mg, prolonged relapse time and
decreased irritability scores. However, increased appetite and weight gain
were common side effects with risperidone, causing two patients to withdraw
from the study prematurely.22
Characteristics of
oppositional defiant disorder (ODD), such as frequent and consistent
temper tantrums or outbursts of rage, can be mistaken as defiant behavior.
Diagnosed as early as age 3 (typically ages 6 to 10), it rarely persists into
adulthood.25 CD arises either before age 10 or between ages 10 and
17 and includes aggressive behavior (e.g., fighting, bullying, physical
assault, intimidation, sexual coercion, and setting fires), nonaggressive
behavior (e.g., vandalism, theft, and deceit), and rules violation (e.g.,
truancy and curfew). For most patients, CD resolves in adulthood, but many are
at risk for substance dependence or abuse and anxiety, mood, and somatoform
disorders.25
Treatment for both CD and ODD
begins with family and individual psychotherapy, involving behaviorial and
environmental changes. Indications for psychotropic medications include
comorbid symptoms. Patients with ODD and ADHD may benefit from atomoxetine
or stimulants, if they are prescribed cautiously.2,13,26 CD
patients with impulsive aggressive behavior may respond to mood stabilizers
(lithium or divalproex), clonidine, or atypical neuroleptics.4,21,24
Self-injurious and stereotypic behaviors respond to antipsychotics.2
A small study involving risperidone demonstrated efficacy in reducing
aggression; olanzepine was shown to be somewhat effective in improving
behavior in a case series; and 40% of patients with moderate
pervasive developmental disorders who took quetiapine showed a decline in
symptoms.27-29 Depression-related aggression that leads to violent
behavior also responds to fluoxetine.
Combination Therapy:
Comorbidities warrant
combination therapy for symptom reduction. For example, adolescents with
bipolar disorder may benefit more from quetiapine and divalproex than from
divalproex alone. In patients with ADHD and bipolar disorder, treatment with a
mood stabilizer increases the efficacy of a stimulant.2 However,
combination therapy may also increase the propensity for side effects. Weight
gain, leading to more severe morbidity, may occur when antipsychotics are
combined with lithium or valproic acid.13
Hypnotics/Anxiolytics
Children and teens who experience
anxiety demonstrate many similarities to adults who suffer from acute and
chronic anxiety disorders.30 An anxiogenic response can be
triggered by a variety of sources and is often associated with the onset of a
chronic anxiety disorder.31 The short-term utilization of sedative
hypnotics and anxiolytics in pediatric care has long been established in a
variety of clinical settings.32 Calming a child with short-acting
benzodiazepines or inhaled nitrous oxide prior to a major surgical procedure,
dental intervention, or diagnostic imaging is safe and effective. Yet, the
best treatment options for chronic pediatric anxiety disorders are still
unclear. A majority of anxiety disorders in children are components or
secondary manifestations of other coexisting disorders, such as depression.
Acceptance of long-term pharmacotherapies with psychotropic agents, such as
SSRIs (fluvoxamine, fluoxetine, and sertraline), high-potency benzodiazepines
(clonazepam), and other agents (with the exception of TCAs), in conjunction
with CBT, has been growing as a therapy option, especially in the setting of
social anxiety disorders.33
CBT
should always be an integral part of therapy. Fluvoxamine is recommended as
the first-line agent initially, at a dose of 25 mg at bedtime for children
ages 8 to 17 years. The dose should be titrated to the lowest effective dose
(range, 50 to 200 mg/day divided twice daily) in 25-mg increments at four- to
seven-day intervals.10 Other SSRIs (e.g., fluoxetine and
sertraline) and mixed reuptake inhibitors (e.g., venlafaxine, mirtazapine, and
bupropion) are acceptable second- and third-line therapy options,
respectively, if fluvoxamine is not effective.
Stimulants and Atomoxetine
Stimulants are the
best-studied psychotropic medications in school-age children and adolescents
with ADHD. The prevalence of ADHD in the school setting averages 6.9% (range,
5.5% to 8.5%); in the community, it is 10.3% (range, 8.2% to 12.7%).
2,34 However, data on dosage for preschool children are still lacking.
Methylpheni date is a dopamine reuptake inhibitor, while amphetamine blocks
norepinephrine and dopamine reuptake. Atomoxetine is a nonstimulant that
inhibits norepinephrine reuptake and is effective in some patients with ADHD,
but its role in children has not been clearly defined.2,35
Clinically, there is no difference in efficacy between the stimulants, but
guidelines recommend switching to a different agent if the response to one
agent is suboptimal. The various dosage forms (immediate-release,
intermediate-release, and delayed-release tablets, patches, and osmotic
technology) offer flexibility in treatment regimens.2,35 Clonidine,
an alpha2 adrenergic agonist, is frequently used to treat rebound
symptoms that occur in the evening; the impulsivity, hyperactivity, and
aggression symptoms of ADHD or another syndrome; or isolated symptoms.2
TCAs or bupropion can be considered if the patient does not improve with
stimulant therapy.
It is well-known that
stimulants may cause linear growth suppression, but the actual frequency and
magnitude of this effect remains unknown.36 In most patients, the
final adult height is reached. Atomoxetine causes minimal projected slowing of
growth in height and weight loss in most patients.37 Height and
weight at baseline should be measured and rechecked annually. Pharmacists can
recommend lowering the dose of the stimulant, placing a child on a drug
holiday, or switching therapy to atomoxetine in children who suffer from
significant growth retardation or weight loss.13,35
Substance-Related
Disorders:
Many adolescents suffer from substance dependence or abuse. In a survey
conducted in 2004, 9% of youths ages 12 to 17 suffer from this condition,
using alcohol, cigarettes, or other illicit drugs. Some researchers have shown
that up to 75% to 80% of adolescents have coexisting mental disorders with
substance dependence or abuse; the most common are CD, ODD, ADHD, affective
disorders, and anxiety disorders. These conditions may have been preexisting
psychiatric disorders that triggered the onset of substance dependence or
abuse. First-line treatment involves cognitive and behavioral programs with
group therapy, family care, and legal, health, recreational, and educational
services. Psychotropic medications are usually prescribed in children who have
coexisting schizophrenia or ADHD. Bupropion is an option for adolescents with
substance dependence or abuse, ADHD, and depression. Stimulants should be
recommended with caution, due to the high abuse potential.38
Lithium can be useful for bipolar disorder and comorbid substance abuse.
Adolescents with depression, anxiety, or substance abuse or dependence would
benefit from an SSRI, not a TCA, which can have anticholinergic and cardiac
side effects.39
Psychiatric Emergencies
An emergencyincludes the onset of suicidality, homicidal or extreme violent urges, perceptual disturbances, disordered thought processes, or a change in cognitive state or abilities. When the child or adolescent has acute or unexplained changes in mental status, treatment with an immediate-acting antipsychotic given intramuscularly, with or without a benzodiazepine, is recommended.11 Olanzapine and ziprasidone are available as intramuscular formulations and are preferred over the older antipsychotics for safety reasons.11
Role of the Pharmacist
The management of mental disorders
can be complicated, and compliance can be compromised if pediatric patients
and their caregivers are not properly educated about disease states and
medications. Judicious selection of psychotropic medications and proper
monitoring are important. While compliance can be enhanced with use of
liquids, orally dissolving tablets, and sustained-release formulations,11
minimizing adverse effects by using the lowest effective dose and proper
monitoring parameters is also essential. Due to all of the negative press
about psychotropic medication use in the pediatric population, it is important
to weigh the benefits against the risks of psychopharmacologic therapy.
References
1. Zito JM, Safer DJ, dosReis S, et al. Trends in the prescribing of psychotropic medications to preschoolers. JAMA.2000;283:1025-1030.
2. Scheffer RE. Psychopharmacology: clinical implications of brain neurochemistry. Pediatr Clin N Am. 2006;54:767-775.
3. Vitiello B. Psychopharmacology for young children: clinical needs and research opportunities. Pediatrics. 2001;108:983-989.
4. Scheffer R, Kowatch R, Carmody T, et al. A randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder following mood stabilization with divalproex sodium. Am J Psychiatry.2005;162:58-64.
5. Shaffer D, Gould MS, Fisher P, et al. Psychiatric diagnosis in child and adolescent suicide. Arch Gen Psychiatry. 1996;53:339-348.
6. Gould MS, King R, Greenwald S, et al. Psychopathology associated with suicidal ideation and attempts among children and adolescents. J Am Acad Child Adolesc Psychiatry. 1998;37:915-923.
7. Fleming JE, Offord DR. Epidemiology of childhood depressive disorders: a critical review. J Am Acad Child Adolesc Psychiatry. 1990;29:571-580.
8. Lewinsohn PM, Clarke GN, Seeley JR, Rohde P. Major depression in community adolescents: Age at onset, episode duration, and time to recurrence. J Am Acad Child Adolesc Psychiatry. 1994;33:809-818.
9. Hoyert DL, Mathews TJ, Menacker F, et al. Annual summary of vital statistics: 2004. Pediatrics. 2006;117:168-183.
10. Taketomo CK, Hodding JH, Kraus DM, eds. Pediatric Dosage Handbook. 12 ed. Hudson, OH: Lexi-Comp Inc., 2005.
11. Scharf MA, Williams TP. Psychopharmacology in adolescent medicine. Adolesc Med.2006;17:165-181.
12. Pagel JF. Medications and their effects on sleep. Prim Care Clin Office Pract. 2005;32:491-509.
13. Correll CU, Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45:771-791.
14. Berkowitz RI, Fabricatore AN. Obesity, psychiatric status, and psychiatric medications. Psychiatr Clin N Am. 2005;28:39-54.
15. Doty RL, Bromley SM. Effects of drugs on olfaction and taste. Otolaryngol Clin N Am. 2004;37:1229-1254.
16. March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA. 2004;292:807-820.
17. Leslie LK, Newman TB, Chesney PJ, et al. The Food and Drug Administration's deliberations on antidepressant use in pediatric patients. Pediatrics. 2005;116:195-204.
18. Kowatch RA, DelBello MP. Pharmacotherapy of children and adolescents with bipolar disorder. Psychiatr Clin N Am. 2005;28:385-397.
19. Calles JL. Psychopharmacology for the violent adolescent. Prim Care Clin Office Pract.2006;33:531-544.
20. Hummel B, Stampfer R, Grunze H, et al. Acute antimanic efficacy and safety of oxcarbamazepine in an open trial with on-off-on design. Bipolar Disord. 2001;3(Suppl 1):43.
21. Wagner KD, Kowatch RA, Emslie GJ, et al. A double-blind, randomized, placebo-controlled trial of oxcarbazepine in the treatment of bipolar disorder in children and adolescents. Am J Psychiatry. 2006;163:1179-1186.
22. Troost PW, Lahuis BE, Steenhuis MP, et al. Long-term effects of risperidone in children with autism spectrum disorders: a placebo discontinuation study. J Am Acad Child Adolesc Psychiatry. 2005;44:1137-1144.
23. American Diabetes Association. American Psychiatric Association. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596-601.
24. Fillipek PA, Accardo PJ, Ashwal S, et al. Practice parameter: screening and diagnosis of autism: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology. 2000;55:468-479.
25. Karnik NS, McMullin MA, Steiner H. Disruptive behaviors: conduct and oppositional disorders in adolescents. Adolesc Med. 2006;17:97-114.
26. Newcorn JH, Spencer TJ, Biederman J, et al. Atomoxetine treatment in children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:240-248.
27. Findling RM, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry. 2000;39:509-516.
28. Soderstrom H, Rastam M, Gillberg C. A clinical case series of six extremely aggressive youths treated with olanzapine. Eur Child Adolesc Psychiatry. 2002;11:138-141.
29. Corson AH, Barkenbus JE, Psey DJ, et al. A retrospective analysis of quetiapine in the treatment of pervasive developmental disorders. J Clin Psychiatry. 2004;65:1531-1536.
30. Pelissolo A, Gourion D, Notides C, et al. Familial factors influencing the consumption of anxiolytics and hypnotics by children and adolescents. Eur Psychiatry. 2001;16:11-17.
31. Hosey MT. Managing anxious children: the use of conscious sedation in paediatric dentistry. Int J Paediatr Dent. 2002;12:359-372.
32. Pollack MH. Comorbidity, neurobiology, and pharmacotherapy of social anxiety disorder. J Clin Psychiatry. 2001;62(Suppl 12)24-29.
33. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. The Research Unit on Pediatric Psychopharmacology Anxiety Study Group. N Engl J Med. 2001;344:1279-1285.
34. Committee on Quality Improvement, Subcommittee on Attention Deficit/Hyperactivity Disorder. American Academy of Pediatrics: Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics. 2000;105:1158-1170.
35. Committee on Quality Improvement, Subcommittee on Attention Deficit/Hyperactivity Disorder. American Academy of Pediatrics: Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics . 2001;108:1033-1044.
36. Poulton A. Growth on stimulant medication; clarifying the confusion: a review. Arch Dis Child. 2005;90:801-806.
37. Spencer TJ, Neweorne JH, Kratochvil CJ et al. Effects of atomoxetine on growth after 2-year treatment among pediatric patients with attention-deficit/hyperactivity disorder. Pediatrics. 2005;116:e74-80.
38. AACAP. Practice parameter for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005;44:609-621.
39. Gee RL, Espiritu RC, Huang LN.
Adolescents with co-occurring mental health and substance use disorders in
primary care. Adolesc Med. 2006;17:427-452.
To comment on this article, contact
editor@uspharmacist.com.






