US Pharm. 2006;7:49-52.
In the United States, approximately 40,000 people are diagnosed with HIV annually, and as a result, AIDS has become one of the leading causes of death in Americans ages 25 to 44 years. Furthermore, an estimated one million people are living with HIV/AIDS in the U.S., while 40 million people are believed to be infected worldwide.1,2
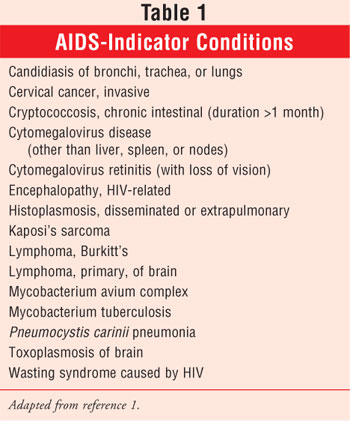
Although it is imperative that people know their HIV/AIDS status, the CDC estimates that nearly one third of Americans infected with HIV/AIDS do not know that they have the disease. Thus, approximately 300,000 people are not receiving therapy and may unknowingly spread the disease through sexual contact or exposure to contaminated blood or from mother to child (e.g., perinatal or vertical transmission or via breast-feeding).2 HIV attacks and kills helper T cells (CD4), resulting in an impaired immune system. If HIV progresses, it may lead to AIDS, which leaves infected people even more susceptible to opportunistic infections.1 See Table 1 for a list of AIDS-indicator illnesses.
Standard HIV testing in the U.S. consists of screening with an enzyme immunoassay (EIA) test and confirming preliminary positive results with a Western blot test. Results are available typically between 48 hours and two weeks, making these assays acceptable when immediate results are not needed. However, even this two-day to two-week window may be problematic: Many patients do not return to their health care providers to receive their test results and thus are oblivious to their HIV/AIDS status.2
HIV Home Testing
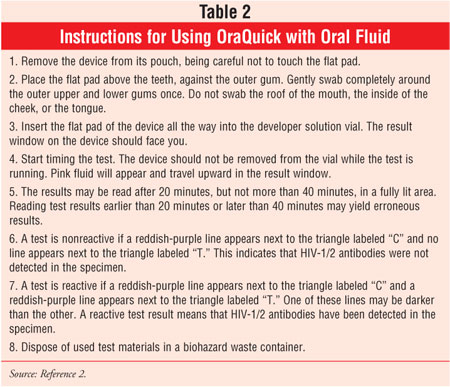
Since 1996, the FDA has approved two home-collection HIV kits that can be purchased OTC. Both tests require individuals to send blood samples to a laboratory and call a toll-free telephone number after a week to anonymously receive results and counseling.3 This idea was taken a step further in November 2005 when OraSure Technologies announced plans to make its rapid HIV test available OTC. The OraQuick Advance Rapid HIV-1/2 Antibody Test, approved by the FDA in 2004 for point-of-care testing of HIV-1/2, differs from other available tests because it is capable of assessing HIV-1/2 antibodies via blood or oral fluid and provides results in 20 minutes.2,3 See Table 2 for instructions on how to use OraQuick.
OraQuick
According to CDC statistics, only one third of patients who test positive for HIV return to their testing site to receive their laboratory results. Furthermore, the use of a rapid HIV test has been shown to increase the number of patients who receive their results, compared to the rates associated with standard HIV testing.4 OraQuick is designed to detect HIV antibodies in oral fluid (saliva), blood samples obtained via finger stick or venipuncture, or plasma samples within 20 minutes.5 In November 2005, OraSure presented the benefits of at-home testing with the nonprescription OraQuick to an FDA advisory committee. The FDA is gathering input from companies, counselors, and public health officials on the safety and feasibility of rapid HIV testing in nonclinical settings.
Clinical Efficacy
The efficacy of OraQuick has been examined in clinical studies. In one study, O'Connell et al. evaluated HIV status as determined by the OraQuick via oral mucosal samples of 100 volunteers who reportedly had a low risk of HIV infection and 101 volunteers who had already tested positive for HIV-1 using standard testing methods. Serum specimens were also evaluated with EIA, and specimens that tested positive were analyzed using Western blot. OraQuick was reactive for 97 of 101 HIV-infected subjects and none of 100 uninfected subjects (P <.001). Of the HIV-positive participants, 90% had been treated with highly active antiretroviral therapy, and 79% were receiving the treatment regimen at the time of the study. Furthermore, the four samples that resulted in false-negatives had a significantly reduced Western blot band as compared to samples from reactive control subjects.6
In a study by Landrum et al., researchers examined the effectiveness of OraQuick in determining the HIV status of individuals who were exposed to HIV due to occupational hazards.7 All positive results as determined by OraQuick were validated using EIA and Western blot. For the 79 specimens in the OraQuick group, the rapid test was 100% concordant with EIA results (CI, 96.3% to 100%). OraQuick was found to be a cost-saving method, as it increased the timeliness of treatment and reduced the level of stress associated with waiting for test results.7
Conclusion
OraQuick has clinically proven accuracy in detecting HIV-1 and -2 in human serum, saliva, and plasma. As studies have indicated that people alter their behavior affecting exposure and risk according to awareness of HIV status, it is possible that availability of an OTC, rapid HIV test such as OraQuick could help reduce HIV transmission, since people may be more likely to test for the disease when the need to visit a clinic is eliminated. Other advantages of OraQuick include noninvasive oral and finger-stick specimen collection and a decreased waiting time for results. However, there are limitations to OraQuick--mainly, the lack of opportunity for counseling that is offered with an ambulatory visit.
References
1. Fletcher C, Kakuda T, Collier A. Human immunodeficiency virus infection. In: DiPiro J, Talbert R, Yee G, et al. Pharmacotherapy: A Pathophysiological Approach. New York, NY: McGraw-Hill; 2002:2151-2174.
2. OraQuick Advance Rapid HIV-1/2 Antibody Test [package insert]. Bethlehem, Pa: OraSure Technologies, Inc.; April 2005.
3. Wright AA, Katz IT. Home testing for HIV. N Engl J Med. 2006;354:437-440.
4. Donovan BJ, Rublein JC, Leone PA, Pilcher CD. HIV infection: point-of-care testing. Ann Pharmacother. 2004;38:670-676.
5. FDA. FDA approves first oral fluid based rapid HIV test kit. Available at: www.cdc.gov/hiv/rapid_testing. Accessed February 9, 2006.
6. O'Connell RJ, Merritt TM, Malia JA, et al. Performance of the OraQuick Rapid Antibody Test for diagnosis of human immunodeficiency virus type 1 infection in patients with various levels of exposure to highly active antiretroviral therapy. J Clin Microbiol. 2003;41:2153-2155.
7. Landrum ML, Wilson CH, Perri LP, et al. Usefulness of a rapid human immunodeficiency virus-1 antibody test for the management of occupational exposure to blood and body fluid. Infect Control Hosp Epidemiol. 2005;26:768-774.
To comment on this article, contact
editor@uspharmacist.com.






