US Pharm. 2017;42(10):12.
Adverse events (AEs) of medications include allergic reactions, side effects, overmedication, and medication errors. AEs are not usually documented at the time a drug comes to market. After the occurrence of an undesirable experience associated with the use of a drug, an AE report is submitted to the FDA.
Contributing Factors: One contributor is the fact that 82% of adults take at least one medication and 29% take five or more. In studies, missed dose (7%), faulty technique (6%), illegible prescription (6%), duplicate therapy (5%), drug-drug interactions (3%-5%), equipment failure (1%), and preparation error (1%) were associated with AEs. Older adults are twice as likely as other patients to visit the emergency department (ED) for AEs (>177,000 visits annually) and seven times as likely to be hospitalized after an emergency visit. Antibiotics are responsible for about one in five (19%) ED visits for AEs. In patients aged 18 years and younger, antibiotics are the most common cause of ED visits for AEs.
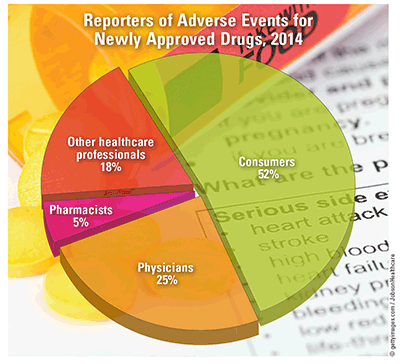
Reporting AEs: Consumers and healthcare providers (HCPs) were almost evenly divided in reporting AEs to the FDA, and the overall rate of reported AEs has increased at a year-over-year rate of 22.9% since 2006. Of all HCPs reporting AEs, 55.7% were physicians, 9.7% were pharmacists, and 34.6% were other HCPs. While the average rate of reporting among physicians and pharmacists decreased by 2.2% and 1.4%, respectively, consumers had a 3.1% average increase in reporting each year. Two-thirds of AE reporters were in the United States; the rest were from foreign countries. On a year-to-year basis over the last decade, domestic reporting increased by an average of 2.4% and foreign reporting decreased by an average of 1.6%. Of all AEs reported in 2016, 95.7% were reported by manufacturers.
Outcomes: Medical errors are the third leading cause of death, claiming 250,000 lives every year, and AEs are one of the major causes of morbidity and mortality in healthcare. The FDA reported that in the first quarter of 2017, of the 297,010 serious outcomes reported, 44,693 deaths occurred. AEs cause more than one million ED visits and 280,000 hospitalizations annually, according to the CDC’s Medication Safety Program, which found that medical costs of AEs total $3.5 billion annually. More than 770,000 people are injured or die each year in hospitals from AEs, costing up to $5.6 million each year per hospital. Patients who experienced AEs were hospitalized an average of 8 to 12 days longer than patients who did not have AEs, and their hospitalization cost $16,000 to $24,000 more.
To comment on this article, contact rdavidson@uspharmacist.com.






