US Pharm. 2006;7:HS30-HS36.
Hospital-acquired pneumonia (HAP) has been
reported as the second most common hospital-acquired infection in the United
States, with an incidence of five to 15 cases per 1,000 hospital admissions.
1-3 HAP accounts for approximately 15% of all hospital-related
infections and may increase a patient's length of hospitalization by four to
nine days.1 Despite the development of new antimicrobials,
mortality rates attributed to HAP are estimated at approximately 33% to 50%,
partly because of controversies surrounding diagnosis and management.2,4,5
HAP is defined as pneumonia that occurs at
least 48 hours after a patient has been admitted to the hospital and that was
not incubating at time of admission. Ventilator-associated pneumonia
(VAP) is a type of HAP in patients receiving mechanical ventilation that
arises at least 48 hours after endotracheal intubation. Health
care–associated pneumonia (HCAP) is a newer term that refers to
pneumonia in any patient who was hospitalized for more than two days and
within 90 days prior to infection; received antibiotic therapy, chemotherapy,
wound care, or chronic dialysis within 30 days prior to infection; or resided
in a long-term care facility or nursing home.2 Despite the various
terminologies used, it is important to note that the same treatment principles
apply to these different classifications of pneumonia.5
The risk of pneumonia is sixfold to 20-fold higher in patients receiving mechanical ventilation, as pneumonia is the most common hospital-acquired infection among this population.1-3,6 VAP is associated with prolonged mechanical ventilation, increased length of stay in the intensive care unit, and increased mortality.1-3,7 Guidelines for the management of immunocompetent adults with HAP, VAP, and HCAP have recently been published through the collaboration of two societies--the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA).2 This article focuses on these guidelines and reviews the current terminology, etiology, pathogenesis, and antimicrobial treatment choices for these types of pneumonia. Although most research has come from the treatment of patients with VAP, this information may be extrapolated and applied to patients with HAP or HCAP.
Diagnosis
The guidelines address two separate diagnostic strategies for HAP, VAP, and HCAP--the clinical strategy and the bacteriologic strategy. Within the clinical strategy, pneumonia is defined as the presence of a new or progressive lung infiltrate on chest radiograph and clinical evidence that the infiltrate is due to infection. Clinical evidence of infection is defined as the presence of at least two of the following symptoms: fever greater than 38°C, leukocytosis or leukopenia, or purulent secretions.2 Additional symptoms of pneumonia may include a change in oxygenation status, a change in mental status, tachypnea, or worsening of an underlying medical illness.8 Using this clinical strategy, the microbial etiology of pneumonia is based on analysis of endotracheal aspirates or sputum with Gram stain and microscopy (to look for the presence of white blood cells, epithelial cells, and the type of bacteria present) and semiquantitative cultures (growth of microorganism[s] described as light/few, moderate, or abundant/many).2 While aspirate and sputum cultures are relatively easy to obtain, their specificity for the diagnosis of pneumonia is low.1
The bacteriologic strategy uses quantitative cultures of samples obtained from the lower respiratory tract by noninvasive methods, such as an endotracheal aspirate, or by invasivemethods, such as a protected specimen brush (PSB) or bronchoalveolar lavage (BAL) to determine the presence of pneumonia and the microorganism(s) responsible for disease. With the bacteriologic strategy, growth that is less than the threshold for each method of sample collection (106 colony-forming units [cfu]/mL for endotracheal aspirates, 103 cfu/mL for PSB, or 104 to 105 cfu/mL for BAL) is assumed to be due to colonization or contamination.2,8 Although use of the bacteriologic strategy improves the specificity for diagnosis of pneumonia, tests performed using this strategy are invasive and have additional complication risks such as hypoxemia, arrhythmias, and bleeding.1
Since a delay in the initiation of appropriate antimicrobial therapy is associated with increased mortality--regardless of the strategy of diagnosis used--empiric antibiotics targeted toward the probable microorganism(s) causing disease should be started in all patients with suspected pneumonia.2
Pathogens
The most common bacterial pathogens associated with HAP, VAP, and HCAP vary depending on the length of time the patient has been hospitalized and/or has received mechanical ventilation, and whether he or she has risk factors for multidrug resistant (MDR) microorganisms (Table 1). Early-onset pneumonia is defined as the occurrence of pneumonia within four days of hospitalization or endotracheal intubation. Late-onset pneumonia is defined as the occurrence of pneumonia five days or longer after hospitalization or endotracheal intubation.2
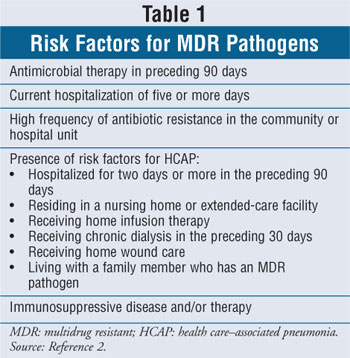
The most common microorganisms causing disease in patients with early-onset HAP or VAP and with no risk factors for MDR organisms include antibiotic-sensitive gram-negative bacilli, such as Escherichia coli, Klebsiella spp., Haemophilus influenzae, Enterobacter spp., Proteus spp., and Serratia marcescens; and gram-positive cocci, such as Streptococcus pneumoniae and methicillin-sensitive Staphylococcus aureus. Microorganisms that cause disease in patients with late-onset HAP or VAP and with risk factors for MDR pathogens include methicillin-resistant S. aureus (MRSA), Pseudomonas aeruginosa, Acinetobacter spp., and Enterobacter spp. 2,3,9,10 The incidence of polymicrobial infections appears to be emerging and may be more common among patients with acute respiratory distress syndrome (ARDS) or late-onset pneumonia.1,2 Although viral and fungal pathogens are rarely isolated in immunocompetent patients, the clinician should consider the possibility that these organisms are causative agents in patients who do not respond to antibiotic therapy or in those who are immunocompromised.2,10
TREATMENT
Empiric Therapy
The ATS/IDSA guidelines support
early initial management of pneumonia based on the time of disease onset and
the patient's risk for MDR pathogens. Early appropriate antibiotic therapy
(defined as the initiation of an antimicrobial agent to which the infecting
microorganism is sensitive within 24 hours of the diagnosis of pneumonia) is
associated with improved patient outcomes.8,11 A review of several
studies found that the use of inappropriate initial antimicrobial therapy for
patients with VAP was associated with a significant increase in mortality.
3 Identifying patients with risk factors for MDR organisms and
classifying disease onset as early or late can help clinicians initiate
appropriate empiric antimicrobial therapy. While these recommendations are a
useful guide, the choice of empiric antibiotic therapy for each patient should
also be determined by a number of factors, including recent or current
antibiotic use, resistance patterns of a specific institution or hospital
unit, side effects, pharmacokinetic and pharmacodynamic properties of
individual agents, and cost.2
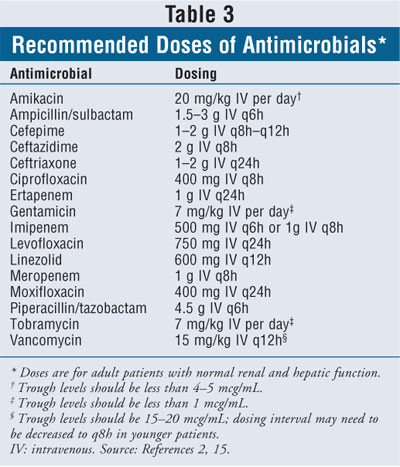
The joint guidelines recommend that patients with early-onset HAP or VAP and no risk factors for MDR pathogens initially be treated with ceftriaxone, ampicillin/sulbactam, ertapenem, or one of the fluoroquinolones, such as levofloxacin, ciprofloxacin, or moxifloxacin (T able 2).2 The appropriate doses of recommended antimicrobial agents are listed in Table 3. Although ciprofloxacin is listed in the guidelines as an option, the authors point out that levofloxacin or moxifloxacin is preferred.2 The use of other fluoroquinolones such as gatifloxacin or gemifloxacin may be appropriate, but their role for the treatment of HAP or VAP has not yet been clearly defined.2
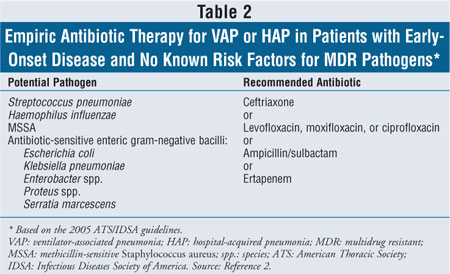
Patients with late-onset pneumonia and/or risk factors for MDR pathogens should initially be treated with at least two antimicrobial agents. The guidelines recommend using two agents with activity against P. aeruginosa--an antipseudomonal cephalosporin (cefepime, ceftazidime), an antipseudomonal carbapenem (meropenem, imipenem), or piperacillin-tazobactam, in combination with an antipseudomonal fluoroquinolone (ciprofloxacin or levofloxacin) or an aminoglycoside (amikacin, gentamicin, or tobramycin). If the patient is at risk for MRSA or if there is a high local rate of MRSA, the guidelines recommend that linezolid or vancomycin also be given (Tables 3, 4).2
Initial treatments should be administered via the
intravenous (IV) route.2,12 In select patients who have a good
clinical response to treatment and a functional gastrointestinal (GI) tract,
medications with good oral bioavailability (e.g., fluoroquinolones, linezolid)
may be switched to oral therapy when appropriate. Usual patient criteria for
conversion from IV to oral route of therapy include clinical improvement
(i.e., white blood cell count decreasing, afebrile), hemodynamic stability,
ability to ingest oral medications, and a functioning GI tract.13
Directed Therapy
Microbiology cultures should be reevaluated and patients should be clinically reassessed after 48 to 72 hours of receiving antimicrobial therapy. If cultures are negative and the patient has shown signs of clinical improvement, the clinician may consider discontinuing antibiotics. If the patient has not shown signs of clinical improvement after 48 to 72 hours of antimicrobial therapy, the clinician should search for other sites of infection or other diagnoses, such as pulmonary embolism, congestive heart failure, ARDS, neoplastic or connective tissue disease, and complications of pneumonia such as empyema or abscess. The clinician may adjust antimicrobial therapy toward other potential pathogens (i.e., opportunistic micro organism, virus, fungus, Mycobacterium tuberculosis, or Legionella spp.).2,11 Once microbiology culture and sensitivity results are available, antibiotic coverage should be de-escalated, and therapy should be directed toward the microorganism(s) causing disease. De-escalation refers to streamlining antimicrobial therapy to an agent that has a more limited spectrum than that used in initial therapy; this is appropriate when an anticipated resistant organism (e.g., P. aeruginosa) is not recovered from a patient or when the isolated organism shows sensitivity to a more narrow-spectrum antimicrobial agent. 2,14
Monitoring the Antimicrobial Regimen
The recommended dosages of antimicrobials for the treatment of HAP, VAP, and HCAP are higher than those routinely used for the treatment of other disease states. The pharmacist should be aware of the potential toxicities of these agents in order to appropriately manage the patient. Patients receiving aminoglycosides are at an increased risk for nephrotoxicity and ototoxicity. Peak and trough levels of these agents, as well as assessment of a patient's auditory and renal function, should be routinely performed. Ceftazidime, fluoroquinolones, and carbapenems may cause seizures in patients who are not appropriately dosed based on renal function. Since piperacillin-tazobactam may cause neutropenia, a complete blood count with differential should be regularly monitored.15
Treatment of Select MDR Pathogens
MRSA: The incidence of pneumonia caused by gram-positive cocci such as S. aureus, including strains that are methicillin-resistant, has been increasing.2 The current recommendation for empiric use of linezolid or vancomycin in patients with risk factors for MRSA comes from two clinical trials that showed linezolid to be equivalent to vancomycin for the treatment of patients with HAP.2,16,17 A combined analysis of these trials showed that the use of linezolid was associated with higher clinical cure and survival rates when compared to vancomycin for the treatment of documented MRSA in patients with VAP.18,19 The current guidelines give the clinician the choice to use either linezolid or vancomycin; it remains controversial if one agent is preferred over the other.20 It is important to note that with use of vancomycin, the guidelines recommend a target trough between 15 and 20 mcg/mL.2
Other antimicrobial agents with activity against MRSA include quinupristin/dalfopristin, daptomycin, and tigecycline. One trial found quinupristin/dalfopristin to be equivalent to vancomycin for the treatment of patients with HAP caused by gram-positive organisms. However, the rates of clinical success in each group were low, and patients who received quinupristin/dalfopristin experienced significantly more frequent venous adverse events.21 Although daptomycin possesses activity against gram-positive microorganisms in vitro, it has poor lung penetration, and its activity has been shown to be inactivated by pulmonary surfactant; thus, it should not be used for the treatment of pneumonia.22 Tigecycline is a recently approved agent that is not currently indicated for the treatment of pneumonia; its role in therapy needs to be defined.23
P. aeruginosa: P. aeruginosa is a difficult microorganism to treat, and controversy exists over the benefit of combination therapy for the treatment of HAP, VAP, or HCAP. Since resistance usually occurs when only one agent is used for the treatment of infections caused by P. aeruginosa, the guidelines recommend combination therapy to avoid ineffective and inappropriate treatment. Combination therapy with an antipseudomonal cephalosporin, an antipseudomonal carbapenem, or piperacillin-tazobactam, plus an antipseudomonal fluoroquinolone or an aminoglycoside in patients with late-onset pneumonia or who are at risk for MDR pathogens, is recommended. Aerosolized antibiotics are not recommended for use as initial therapy but may be considered by the clinician for use as adjunctive therapy in patients with a resistant microorganism who do not respond to systemic agents.2
Acinetobacter species: Acinetobacter is also a difficult microorganism to treat because of its inherent resistance to several antimicrobial agents. The guidelines suggest the use of a carbapenem or ampicillin/sulbactam (because of the activity of the sulbactam component against this microorganism). For select patients with isolates resistant to the aforementioned agents, polymyxin or colistin may be considered.2
Gram-Negative Microorganisms that Produce Extended-Spectrum Beta-Lactamase
The incidence of disease caused by microorganisms that produce extended-spectrum beta-lactamase (ESBL) has also been increasing. The most common ESBL-producing organisms are members of the Enterobacteriaceae family. Although microbiology laboratories routinely test Klebsiella spp. and E. coli for the presence of this enzyme, an institution's specific laboratory may not look for its presence in other gram-negative isolates. These ESBL-producing organisms are usually resistant to most beta-lactam antibiotics, aminoglycosides, and fluoroquinolones. Carbapenems and cephamycins, such as cefoxitin or cefotetan, may retain activity against these types of microorganisms. The guidelines recommend a carbapenem as the most reliable antimicrobial agent for the treatment of a suspected or documented ESBL-producing microorganism. The roles of the fourth-generation cephalosporin (cefepime), piperacillin-tazobactam, and combination therapy have not yet been clearly defined.2
Duration of Treatment
VAP has traditionally been treated with a 14- to
21-day course of antimicrobials. Newer data suggest that the total duration of
therapy for uncomplicated HAP, VAP, and HCAP may be reduced to approximately
seven to eight days in patients who initially received an appropriate
antibiotic regimen, are showing resolution of clinical features of infection,
and do not have pneumonia caused by a nonfermenting gram-negative bacillus
such as P. aeruginosa, Stenotrophomonas maltophilia, or
Acinetobacter spp. In one randomized trial of patients with VAP, patients
who received eight days of antimicrobials had fewer recurrences and less
resistance than those who received 15 days of antibiotics. Although there was
no difference in mortality between the two groups, patients who were infected
with a nonfermenting gram-negative bacillus and received a shorter treatment
course were more likely to have a disease relapse. If a shorter course of
therapy is chosen, patients should be closely monitored for relapse of
pneumonia after antibiotics have been discontinued.2,8,24
If patients were initially receiving
combination therapy with a regimen containing an aminoglycoside, the
aminoglycoside may be discontinued after five to seven days in patients who
are responding to treatment. Select patients may be transitioned from IV to
oral agents to complete the course of therapy.2
Conclusion
Clinicians should be aware of the recent guidelines for the treatment of HAP, VAP, and HCAP. Since a delay in the initiation of appropriate antimicrobial therapy is associated with increased mortality, pharmacists should be able to recommend an empiric antimicrobial regimen based on patient-specific factors. Appropriate empiric and directed antimicrobial therapy may help to lower the high morbidity and mortality rates associated with these diseases.
References
1. Tablan OC, Anderson LJ, Besser R, et al. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee: guidelines for preventing health-care–associated pneumonia, 2003. MMWR Recomm Rep. 2004;53(RR-3):1-36.
2. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
3. Höffken G, Niederman MS. Nosocomial pneumonia: the importance of a de-escalating strategy for antibiotic treatment of pneumonia in the ICU. Chest. 2002;122:2183-2196.
4. Dugan HA, MacLaren R, Jung R. Duration of antimicrobial therapy for nosocomial pneumonia: possible strategies for minimizing antimicrobial use in intensive care units. J Clin Pharm Ther. 2003;28:123-129.
5. Grossman RF, Rotschafer JC, Tan JS. Antimicrobial treatment of lower respiratory tract infections in the hospital setting. Am J Med. 2005;118(suppl 7A):29S-38S.
6. Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115-2121.
7. Fowler RA, Flavin KE, Barr J, et al. Variability in antibiotic prescribing patterns and outcomes in patients with clinically suspected ventilator-associated pneumonia. Chest. 2003;123:835-844.
8. Craven DE, Palladino R, McQuillen DP. Healthcare-associated pneumonia in adults: management principles to improve outcomes. Infect Dis Clin North Am. 2004;18:939-962.
9. Shaw MJ. Ventilator-associated pneumonia. Curr Opin Pulm Med. 2005;11:236-241.
10. McCrory R, Jones DS, Adair CG, Gorman SP. Pharmaceutical strategies to prevent ventilator-associated pneumonia. J Pharm Pharmacol. 2003;55:411-428.
11. Iregui M, Ward S, Sherman G, et al. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122:262-268.
12. Fagon JY, Chastre J. Antimicrobial treatment of hospital-acquired pneumonia. Clin Chest Med. 2005;26:97-104.
13. Mandell LA, Bartlett JG, Dowell SF, et al. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin Infect Dis. 2003;37:1405-1433.
14. Rello J, Vidaur L, Sandiumenge A, et al. De-escalation therapy in ventilator-associated pneumonia. Crit Care Med . 2004;32:2183-2190.
15. Lacy CF, Armstrong LL, Goldman MP, Lance LL. Drug Information Handbook. 14th ed. Hudson, Ohio: Lexi-Comp; 2006.
16. Rubinstein E, Cammarata S, Oliphant T, et al. Linezolid (PNU-100177) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin Infect Dis. 2001;32:402-412.
17. Wunderink RG, Cammarata SK, Oliphant TH, et al. Continuation of a randomized, double-blind, multicenter study of linezolid versus vancomycin in the treatment of patients with nosocomial pneumonia. Clin Ther. 2003;25:980-992.
18. Wunderink RG, Rello J, Cammarata SK, et al. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124:1789-1797.
19. Kollef MH, Rello J, Cammarata SK, et al. Clinical cure and survival in Gram-positive ventilator-associated pneumonia: retrospective analysis of two double-blind studies comparing linezolid with vancomycin. Intensive Care Med. 2004;30:388-394.
20. Bauer T. Nosocomial pneumonia: therapy is just not good enough. Chest. 2003;124:1632-1634.
21. Fagon J, Patrick H, Haas DW, et al. Treatment of gram-positive nosocomial pneumonia: prospective randomized comparison of quinupristin/dalfopristin versus vancomycin. Am J Respir Crit Care Med. 2000;161(3 pt 1):753-762.
22. Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149-2152.
23. Tygacil (tigecycline) product information. Philadelphia, PA: Wyeth. May 2006.
24. Chastre J, Wolff M, Fagon JY, et al.
Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated
pneumonia in adults: a randomized trial. JAMA. 2003;290:2588-2598.
To comment on this article, contact
editor@uspharmacist.com.






