US Pharm.
2007;32(10):HS-3-HS-13.
New molecular entities (NMEs), as defined by
the FDA, are new drug products that have as their active ingredient a chemical
substance marketed for the first time in the United States. The following
descriptions of the NMEs approved during the first half of 2007 detail the
pharmacotherapeutic design and mechanism of action of each new drug.
Also included is a summary of selected clinical data presented to the FDA in
support of the manufacturer's new drug application (NDA). The FDA classifies
NMEs on the basis of therapeutic potential (Table). NMEs classified as
priority review (P) represent significant improvement in comparison to
marketed products in the treatment, diagnosis, or prevention of a disease.
NMEs receiving standard review (S) are those that appear to have therapeutic
qualities similar to those of one or more already marketed drugs.
This review is intended to be objective
rather than evaluative in content. The information for each reviewed NME was
obtained primarily from sources published prior to FDA approval. Experience
clearly demonstrates that many aspects of a new drug's therapeutic profile,
not detected in premarketing studies, surface after the drug is used in large
numbers of patients. Studies have indicated the appearance of "new" adverse
reactions for many NMEs within two to three years of the drug becoming
available. Many of these drugs may eventually acquire at least one black box
warning for serious adverse drug reactions or are withdrawn from the market
for safety reasons that were not recognized at the time of approval. Hence,
while this review offers a starting point for learning about new drugs, it is
essential that practitioners be vigilant of changes in a drug's therapeutic
profile as reported by their own patients and in the pharmaceutical literature.
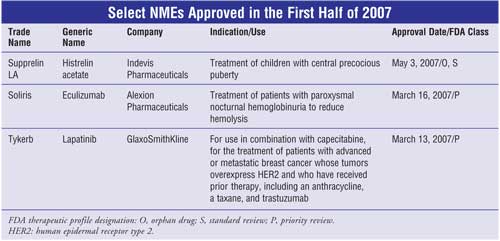
Histrelin Acetate (Supprelin LA, Indevis
Pharmaceuticals)
Indication and Clinical
Profile:1-4 Central precocious puberty (CPP) is the premature
development of body characteristics that normally occur during puberty. In
females, this is usually defined as younger than 8 years, and in males,
younger than 9 years. Signs of early puberty include breast enlargement in
girls and the appearance of hair in the genital area in boys and girls.
Children with CPP also show significantly advanced bone age that can result in
diminished adult height attainment as well as an increased likelihood of
psychosocial problems. About 6,000 American children have this condition, with
2,000 new cases diagnosed each year.
Histrelin acetate is indicated
specifically for the treatment of children with CPP and was developed as an
orphan product. Orphan status provides incentives for companies to develop
products for use in conditions that afflict fewer than 200,000 people
annually. FDA approval was based on the results of two open-label, single-arm
clinical studies (study 1 and study 2). Study 1 enrolled 11 pretreated female
subjects, ages 3.7 to 11 years. Study 2 enrolled 36 subjects (33 females and
three males), ranging in age from 4.5 to 11.6 years. Sixteen of these subjects
were pretreated, and 20 were treatment naïve. End points were similar in both
trials and included measurements of the suppression of gonadotropins
(luteinizing hormone [LH] and follicle-stimulating hormone [FSH]) and gonadal
sex steroids (estrogen in girls, testosterone in boys). Additional assessments
were clinical (evidence of stabilization or regression of signs of puberty) or
gonadal steroid dependent (bone age, linear growth). In study 2, the primary
measure of efficacy was LH suppression, defined as a peak LH of <4 mIU/mL
following stimulation with the gonadotropin-releasing hormone (GnRH) analog
leuprolide acetate. In both trials, the end points were achieved. In study 2,
suppression of LH was induced in all treatment-naïve subjects and maintained
in all pretreated subjects one month after implantation and continued through
month 12. Secondary efficacy assessments indicated stabilization of disease.
In addition, in study 2, estradiol suppression was present in 100% of the
females through month 9 and was 97% at month 12. Testosterone suppression was
maintained in the three pretreated males.
Pharmacology and
Pharmacokinetics:1,2 Continuous administration of the GnRH
agonist histrelin acetate causes a reversible down-regulation of the GnRH
receptors in the pituitary gland and desensitization of the pituitary
gonadotropes. These inhibitory effects result in decreased levels of LH and
FSH that, in turn, cause a reduction in ovarian (estrone and estradiol) and
testicular (testosterone and dihydrotestosterone) steroidogenesis. Based on
this mechanism, initial exposure to histrelin acetate results in a transient
increase in circulating levels of LH and FSH and in the concentration of
gonadal steroids.
In clinical trials, implantation of
histrelin acetate provided average maximum serum histrelin concentrations of
0.43 ng/mL--a level expected to maintain gonadotropins at prepubertal levels.
There was no apparent pharmacokinetic difference between naïve subjects who
received a luteinizing hormone–releasing hormone (LHRH) agonist and subjects
previously treated with an LHRH agonist.
Adverse Reactions and Dug
Interactions:1-4 During the early phase of histrelin acetate
therapy, gonadotropins and sex steroids rise above baseline because of the
natural stimulatory effect of the drug (see Pharmacology section). Therefore,
an increase in clinical signs and symptoms may be observed initially. The most
commonly reported adverse reaction (occurring in about 50% of patients) was
implant site reactions, including scarring, bruising, soreness, pain,
tingling, itching, and swelling. In 22% of the girls, light vaginal bleeding
occurred during the first month. Other infrequent effects included headache
and nausea and vomiting. Although not reported with histrelin acetate, serious
and life-threatening allergic reactions have occurred with GnRH. No formal
drug–drug, drug–food, or drug–herb interaction studies were performed. Results
of diagnostic tests of pituitary gonadotropic and gonadal functions conducted
during and after histrelin acetate therapy may be affected.
Dosage and Administration:
1 Histrelin acetate is supplied as a sterile, nonbiodegradable,
diffusion-controlled reservoir drug delivery system (hydron implant
technology) that contains 50 mg of active drug. The implant is inserted
subcutaneously in the inner aspect of the upper arm and delivers approximately
65 mcg of histrelin acetate per day over 12 months. The implant must be
removed after 12 months of therapy. (The implant has been designed to allow a
few additional weeks of histrelin acetate release in order to enable
flexibility of medical appointments.) When an implant is removed, another
implant may be inserted to continue therapy. Discontinuation of therapy should
be considered at the discretion of the physician and at the appropriate time
point for the onset of puberty (about age 11 for females and age 12 for males).
Counseling Points and
Precautions:1 Histrelin should not be used in children younger
than 2 years or in women who are or may become pregnant, since the drug may
cause harm to the fetus. Patients who receive the histrelin implant should
keep the arm clean and dry, should not swim or bathe for 24 hours, and avoid
heavy play or exercise that uses the implanted arm for seven days. After the
implant site incision has healed, patients may return to normal activities.
After 12 months, the implant must be removed and a new implant may be inserted
to continue treatment. It may be necessary to perform an ultrasound or MRI to
locate the implant prior to removal.
Eculizumab (Soliris, Alexion
Pharmaceuticals)
Indication and Clinical
Profile:5-7 Paroxysmal nocturnal hemoglobinuria (PNH) is a rare
blood disorder that occurs due to hematopoietic stem cell mutation, resulting
in a lack of protective complement-regulating surface membrane proteins on red
and white blood cells as well as on platelets. Patients with PNH are unable to
synthesize the glycosyl-phosphatidylinositol anchor glycolipid that binds
surface proteins to cell membranes and protects the cell from destructive
substances called terminal complement. The disease causes the hemolysis
of blood cells resulting in dark urine, hemolytic anemia, pancytopenia,
pulmonary hypertension, and thrombosis of large veins typically hepatic,
abdominal, cerebral, and subdermal. PNH is not inherited, affects men and
women of all races equally, and can occur at any age (median age of diagnosis,
35 to 40 years). The median survival is about 10.3 years, with some patients
surviving up to 25 years and documented reports of spontaneous remission.
Until recently, PNH was treated with symptom management with transfusions and
supplements for anemia, anticoagulants for thrombosis, immunosuppressants or
steroids for low cell counts, and bone marrow transplantion. Eculizumab
(Soliris) was approved in March 2007 for the treatment of PNH to reduce
hemolysis. It is the first complement inhibitor approved in the U.S.
Pharmacology and
Pharmacokinetics:5,6 Eculizumab is a recombinant
humanized monoclonal IgG2/4-kappa antibody that binds specifically
with high affinity to the complement protein C5. Binding to C5 inhibits its
cleavage to C5a and C5b proteins, preventing the generation of the terminal
complement complex C5b-9 and inhibiting intravascular hemolysis. The clearance
of eculizumab in a typical 70-kg PNH patient is 22 mL/hour, with a volume of
distribution of 7.7 L and an average half-life of 272 hours. The mean peak and
trough serum concentrations were 194 and 97 mcg/mL, respectively.
Adverse Reactions and Drug
Interactions:5 In clinical trials, the most significant adverse
event in terms of severity in patients receiving eculizumab was meningococcal
infections, with two patients experiencing meningococcal sepsis. Overall, the
most common adverse events (? 10% overall and greater than placebo)
occurring in clinical trials included headache, nasopharyngitis, back pain,
and nausea. Serious adverse reactions occurred in 9% of the treated patients
in the placebo-controlled study. These reactions included progression of PNH
and infections. Approximately 16% of patients in the single-arm and long-term
exposure study experienced serious adverse events, which included viral
infection, headache, anemia, and pyrexia. No drug interaction studies have
been performed with eculizumab to date.
Dosage and Administration:
5,7 Eculizumab is supplied in a 300-mg, single-use vial containing
30 mL of 10 mg/mL sterile solution that must be refrigerated and protected
from light until time of use. Patients treated with eculizumab must receive a
meningococcal vaccine at least two weeks before the initiation of eculizumab
therapy and revaccinated according to current guidelines. The eculizumab
solution must be diluted with the appropriate diluent to a final admixture
concentration of 5 mg/mL before intravenous (IV) infusion. It may not be
administered as an IV push or bolus injection. The final 5 mg/mL infusion
volume is 120 mL for a 600-mg dose or 180 mL for a 900-mg dose. The admixture
should be allowed to adjust to room temperature and be visually inspected for
particles or discoloration before being administered. It is stable at room
temperature for 24 hours after preparation. The recommended eculizumab dosage
is 600 mg every seven days for the first four weeks, followed by 900 mg for
the fifth dose seven days later, then 900 mg every 14 days thereafter. The
admixture should be administered by the recommended time points or within two
days of each time point. The infusion should occur over 35 minutes. It may be
stopped or slowed if adverse reactions occur, but total infusion time should
not exceed two hours. After the completion of the infusion, the patient should
be monitored for at least one hour for signs and symptoms of an infusion
reaction. If therapy is discontinued, patients should be monitored for eight
weeks for serious hemolysis and serum lactate dehydrogenase levels.
Precautions and Counseling
Points:5 The use of eculizumab is contraindicated in patients
with unresolved serious Neisseria meningitidis infection and in
patients who are not currently vaccinated against N meningitidis.
Meningococcal infections may still occur even with vaccination. Patients
should be monitored for early signs and symptoms of meningococcal infections
and be treated with antibiotics if necessary. Discontinuation of eculizumab
therapy should be considered during the treatment of serious meningococcal
infections. Caution should be exercised when using eculizumab in patients who
have any systemic infection. Eculizumab is FDA pregnancy category C and should
be used during pregnancy only if the potential benefit outweighs the potential
risk to the fetus. It is not known whether eculizumab is excreted in breast
milk, and caution should be used when administered to nursing women. The
safety and efficacy of eculizumab in children younger than 18 years have not
been established.
Lapatinib (Tykerb,
GlaxoSmithKline)
Indication and Clinical
Profile:8-11 Lapatinib is indicated specifically in combination
with capecitabine for the treatment of patients with advanced or metastatic
breast cancer whose tumors overexpress human epidermal receptor type 2 (HER2)
and who have received prior therapy, including an anthracycline, a taxane, and
trastuzumab. FDA approval was based on the results of one clinical trial
involving 399 subjects with tumors overexpressing HER2 in locally advanced or
met a static breast cancer that was progressing after prior
treatment, which included anthracyclines, taxanes, and trastuzumab.
Subjects were randomized to receive either lapatinib 1,250 mg once daily
(continuously) plus capecitabine 2,000 mg/m2/day on days 1 to 14
every 21 days, or to receive capecitabine alone at a dosage of 2,500 mg/m2
/day on days 1 to 14 every 21 days. The primary end point was time to
progression (TTP), defined as time from randomization to tumor progression or
death related to breast cancer. The median TTP was 23.9 weeks for the
combination treatment, compared to 18.3 weeks for capecitabine alone, for a
response rate of 31.8% versus 17.4%, respectively.
Pharmacology and
Pharmacokinetics:8,9 Tyrosine kinases are enzymes that provide
a central switch mechanism in cellular signal transduction pathways and as
such are involved in many cellular processes (e.g., cell proliferation,
metabolism, survival, and apoptosis). Several specific tyrosine kinases are
known to be activated in cancer cells and to drive tumor growth and
progression. Blocking tyrosine kinase activity therefore represents a rational
approach to cancer therapy. Therapeutic strategies include blocking
kinase-substrate interaction, inhibiting the enzyme's adenosine
triphosphate–binding site, and blocking extracellular tyrosine kinase
receptors on tumor cells. Several tyrosine kinase inhibitors (TKIs), including
gefitinib (Iressa) and trastuzumab (Herceptin), have been approved as
anticancer agents.
The erbB (or HER) family of
transmembrane tyrosine kinase receptors, especially receptors erbB1 (or
epidermal growth factor receptor [EGFR]) and erbB2 (or HER2), has been
identified as an important therapeutic target in a number of cancers. HER2,
for example, is overexpressed in roughly 20% to 30% of patients with
aggressive breast cancer, while EGFR is overexpressed in several solid tumors.
Lapatinib is an orally active TKI that targets both erbB1 and erbB2 receptors.
Its dual mode of action differs from existing TKIs, such as gefitinib and
trastuzumab, which are single EGFR and HER2 receptor inhibitors, respectively.
It is hoped that dual TKIs may help to address the problem of drug resistance
that can arise following treatment with single-receptor inhibitors.
Lapatinib is incompletely and
variably absorbed from the gastrointestinal tract. Peak plasma concentrations
(Cmax) are achieved approximately four hours after administration, and steady
state is achieved within six to seven days with repeated dosing. Lapatinib AUC
(area under the curve) values are approximately three- and fourfold higher,
and Cmax is approximately 2.5- and threefold higher when administered with a
low-fat (5% fat, 500 calories) or high-fat (50% fat, 1,000 calories) meal,
respectively. Lapatinib is highly bound (>99%) to albumin and alpha-1 acid
glycoprotein. The drug is a substrate for the transporters breast cancer
resistance protein (BCRP, ABCG2) and P-glycoprotein (Pgp, ABCB1). Lapatinib
has also been shown in vitro to inhibit these efflux transporters, as well as
the hepatic uptake transporter OATP 1B1.
Lapatinib undergoes extensive
metabolism, primarily by cytochrome P-450 (CYP) 3A4 and CYP3A5, with minor
contributions from CYP2C19 and CYP2C8 to a variety of oxidation metabolites.
None of these metabolites account for more than 14% of the dose recovered in
the feces or more than 10% of lapatinib concentration in plasma. The terminal
phase half-life following a single dose is 14.2 hours; accumulation with
repeated dosing indicates an effective half-life of 24 hours. Elimination of
lapatinib is predominantly through metabolism by CYP3A4/5 with negligible
(<2%) renal excretion. Recovery of parent drug in feces accounts for a median
of 27% (range, 3% to 67%) of an oral dose. The effects of age, gender, or race
on the pharmacokinetics of lapatinib have not been investigated.
Adverse Reactions:9-11
In clinical trials, adverse events leading to discontinuation were similar in
the lapatinib-capecitabine combination arm versus capecitabine alone, with a
rate of 14%. Most commonly reported adverse effects were gastrointestinal
(diarrhea, nausea, and vomiting), dermatologic (palmar-plantar
erythrodysesthesia and rash), and fatigue. Diarrhea was the most common
adverse reaction, resulting in discontinuation.
Due to potential cardiac
toxicity with HER2 (erbB2) inhibitors, left ventricular ejection fraction
(LVEF) was monitored in clinical trials at about eight-week intervals. LVEF
decreases were defined as signs or symptoms of deterioration in left
ventricular cardiac function that are grade 3 or higher based on the National
Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) or
as a ? 20% decrease in LVEF relative to baseline, which is below the
institution's lower limit of normal. Among 198 patients who received
lapatinib/capecitabine treatment, three experienced grade 2 and one had grade
3 LVEF adverse reactions.
Drug Interactions:9-11
Concomitant administration of lapatinib with capecitabine did not
substantially alter the pharmacokinetics of either agent (or the metabolites
of capecitabine). Lapatinib undergoes extensive metabolism by CYP3A4. Thus,
concomitant administration of strong inhibitors (e.g., ketoconazole) or
inducers (e.g., carbamazepine) of CYP3A4 alter lapatinib concentrations
significantly. Concurrent administration of ketoconazole resulted in about a
3.6-fold increase in AUC, and the half-life increased to 1.7-fold of control.
Concomitant use of the CYP3A4 inducer carbamazepine resulted in a 72%
reduction in lapatinib AUC. Thus, dosage adjustment of lapatinib should be
considered for patients who must receive concomitant therapy with strong
inhibitors or strong inducers of CYP3A4 enzymes.
Lapatinib inhibits CYP3A4 and
CYP2C8 in vitro at clinically relevant concentrations. Thus, caution is
advised, and dosage reduction of the concomitant substrate drug should be
considered when dosing lapatinib concurrently with medications with narrow
therapeutic windows that are substrates of CYP3A4 or CYP2C8. Lapatinib does
not appear to significantly inhibit CYP1A2, CYP2C9, CYP2C19, CYP2D6, or UGT
enzymes. Since lapatinib is a substrate of the efflux transporter Pgp, ABCB1;
thus, caution should be exercised if it is administered with other drugs that
inhibit Pgp (e.g., ritonavir, cyclosporine, verapamil).
Dosage and Administration:
9 Lapatinib is supplied as a 250-mg tablet. The recommended initial
dosage is 1,250 mg (five tablets) orally once daily on days 1 to 21
continuously in combination with capecitabine 2,000 mg/m2/day
(administered orally in two doses approximately 12 hours apart) on days 1 to
14 in a repeating 21-day cycle. Dividing the daily dose is not recommended.
The drug should be taken on an empty stomach, at least one hour before or one
hour after eating. Lapatinib should be discontinued in patients with decreased
LVEF that is grade 2 or greater according to the NCI CTCAE scale and in
patients with an LVEF that drops below the institution's lower limit of
normal. Treatment may be restarted at a reduced daily dose (1,000 mg) after a
minimum of two weeks if the LVEF recovers to normal and the patient is
asymptomatic. Patients with severe hepatic impairment should have their dosage
reduced. The concomitant use of potent CYP3A4 inhibitors and inducers should
be avoided. If a grade 2 NCI CTCAE toxicity occurs, discontinuation or
interruption of lapatinib may be considered. Treatment can resume at 1,250
mg/day when toxicity improves to grade 1 or less.
Counseling Points and
Precautions:9-11 Before initiating lapatinib, patients should
be assessed to determine if they have heart disease, liver disease, or a
history of long QT syndrome. Patients should also be asked if they are using
antibiotics, ulcer medications, seizure medications, HIV or AIDS medications,
herbal supplements, heart or blood pressure medication, or an antidepressant.
Lapatinib is FDA pregnancy category D and thus should not be used during
pregnancy. Patients are advised to use an effective form of birth control
while taking this medication. It is not known whether lapatinib passes into
breast milk or if it could harm a nursing baby. Therefore, caution is advised
in breast-feeding. Patients should be counseled to stop using the drug and
call their physician if they have serious side effects, such as severe
diarrhea or vomiting or uneven heart rate with extreme dizziness or fainting.
References
1. Hirsch HJ,
Gillis D, Strich D, et al. The histrelin implant: a novel treatment for
central precocious puberty. Pediatrics. 2005;116:798-802.
2. Supprelin LA
[package insert]. Lexington, MA: Indevus Pharmaceuticals, Inc.; 2007.
3. Eugster EA, Clarke
W, Kletter GB, et al. Efficacy and safety of histrelin subdermal implant in
children with central precocious puberty: a multicenter trial. J Clin
Endocrinol Metab. 2007;92:1697-1704.
4. Feuillan PP, Jones
JV, Barnes K, et al. Follow-up of children and young adults after GnRH-agonist
therapy or central precocious puberty. J Endocrinol Invest.
2001;24:734-736.
5. Soliris [package
insert]. Cheshire, CT: Alexion Pharmaceuticals Inc.; 2007.
6. Hillmen P, Young NS,
Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal
hemoglobinuria. N Eng J Med. 2006;355:1233-1243.
7. Besa EC, Talavera F,
Conrad M, et al. Paroxysmal nocturnal hemoglobinuria. eMedicine from WebMD,
May 18, 2007. Available at: www.emedicine.com/med
/topic2696.htm#section~Followup.
8. Montemurro F,
Valabrega G, Aglietta M. Lapatinib. A dual inhibitor of EGFR and HER2 tyrosine
kinase activity. Expert Opin Biol Ther. 2007;7:257-268.
9. TYKERB [package
insert]. Research Triangle Park, NC: Glaxo SmithKline; 2007.
10. Geyer CE, Forster
J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced
breast cancer. New Engl J Med. 2006;355:2733-2743.
11. Konecny GE, Pegram
MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib
(GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer
cells. Advances Cancer Res. 2006;66:1630-1639.
To comment on this article,
contact editor@uspharmacist.com.






