US Pharm
. 2006;6:HS-26-HS-36.
Urinary tract infection (UTI)--a broad term
used to describe bacterial infection of the urethra, bladder, and kidneys--is a
problem frequently encountered by health care providers today. In addition to
bacteria, viruses and fungi are other infectious agents that colonize the
urinary tract. Traditionally, UTIs are categorized as uncomplicated or
complicated, or by site of infection. Infections may be symptomatic or
asymptomatic. Lower UTIs include urethritis and cystitis, and upper tract
infections include pyelonephritis and renal abscesses. Acute infections are
usually associated with a single pathogen; chronic infections are usually
polymicrobial.
The economic impact of bacterial UTIs is a
major factor affecting health care expenses today. Both outpatient and
inpatient treatment contribute to overall costs. The urinary tract is the most
common site of hospital infection, accounting for more than 40% of nosocomial
infections (estimated to be 600,000 patients per year) reported by acute care
hospitals.1 The vast majority of hospital-acquired infections are
due to indwelling catheters. On average, a hospital-acquired UTI increases
length of stay by one day, resulting in nearly one million extra hospital
days. The economic impact is between $424 million and $451 million annually.
2 UTI accounts for approximately eight million health care provider
visits in the United States. More than 100,000 hospitalizations per year are
due to infections of the urinary tract. Uncomplicated cystitis is by far the
most common outpatient infection, while pyelonephritis accounts for the
majority of inpatient visits.3 Diagnosis of UTIs accounts for an
estimated $6 billion in health care expenditures.4
Epidemiology
Nearly half of all women will
have a UTI once in their lives.5 It has been reported that one
third of women will have had at least one UTI by age 24 years.6
Simple, uncomplicated UTIs are quite common in women ages 20 to 50. The
geriatric community is frequently affected by these infections. Notably, these
infections often do not cause symptoms.7 UTIs are the second most
common type of infection in the geriatric population, accounting for nearly
25% of all infections in the elderly.6 Fifty percent of elderly
women are affected by asymptomatic bacteriuria. In many cases, bladder
catheriterization is a contributing factor; 38% of chronic care residents
require bladder catheterization, a cause for the increasing incidence of UTIs
in the elderly.8
The pediatric population is also affected by
UTIs. Bacteriuria is present in 2.7% of boys and 0.7% of girls.9
Uncircumcised males have a higher incidence of infection. Uncircumcised
infants younger than 6 months have a higher incidence of gram-negative
uropathogens.6 The rate of hospital admission is higher in
uncircumcised boys, since this population has a 12-fold increased UTI risk.
10 A study by Nuutinen and Uhari found that 35% of boys and 32% of girls
who had their first UTI before age 1 contracted a recurrent UTI during the
next three years.11 Other risk factors for exposure in infants are
hospitalization and catheterization. Children between ages 1 and 5 years have
a 4.5% increased incidence of bacteriuria.
Pathophysiologic Considerations
Transitional epithelium
transports urine from the kidneys to an elastic bladder. The bladder stores
large volumes of urine at low pressures.12 The incidence of
cystitis is greater in women, primarily due to the proximity of the urethral
opening to the vagina and perianal area. Risk factors for UTIs in women
include fecal contamination, recent UTI, decreased fluid intake, irregular
emptying, sexual intercourse, diaphragm and/or spermicide use, a symptomatic
partner, pregnancy, menopause, low vaginal pH or dryness of mucosa, neurogenic
bladder, renal disease, urologic anatomic abnormalities, instrumentation,
immunosuppression, hospitalization, nephrolithiasis, and diabetes.5
UTIs are less likely to occur in men under
50 years of age. Unlike female anatomy, the male urethra is separated from the
rectum by several centimeters and is keratinized by squamous epithelium. The
incidence of UTI becomes similar in men and women at age 65, given the
increased proportion of men with benign prostatic hypertrophy.12
Approximately one fifth of men in their 70s have experienced a UTI.13
Other risk factors for men include urologic abnormalities, neurogenic
bladder, instrumentation, anal intercourse, and immunosuppression.5
Immunosuppressed states in males and females
include age, diabetes, multiple sclerosis, HIV, and other chronic illnesses
that may impair the immune system. For the diabetic patient, the risk of UTI
is greater in females than in males. Diabetic patients generally seem to have
a twofold to fourfold increased incidence of bacteriuria,13 leading
to a higher incidence of pyelonephritis.
Symptoms of UTI are similar among the sexes:
Dysuria (painful urination), urgency, hesitancy, polyuria, and incomplete
voiding may all be associated with acute cystitis. Urinary incontinence,
hematuria, and suprapubic or low back pain may also be present. Typically,
females with acute dysuria have one of three types of infections: acute
cystitis; acute urethritis due to Chlamydia trachomatis, Neisseria
gonorrheae, or herpes simplex virus; or vaginitis due to Candida or
Trichomonas.
Symptoms that indicate pyelonephritis
include fever, costovertebral angle pain, nausea, and vomiting. Hematuria may
occur in any UTI, but it is more suggestive of nephrolithiasis when
accompanied by flank pain.
In the pediatric population during the first
8 to 12 weeks of life, UTI may be associated with bacteremia. Symptoms in
infants up to 2 years old may include difficulty with feeding, nausea and
vomiting, or failure to thrive. Children ages 2 to 5 may demonstrate fever and
abdominal pain. Children under 5 are at risk for renal scarring.14
Children older than 5 may have the same symptomatology as adults. As many as
25% of young children without pyelonephritis have renal bacteriuria.15
As in other age groups, urine culture is the goal standard for diagnosis of
UTI. However, an adequate sterile urine culture is often difficult to obtain
in the pediatric patient. The urinalysis will support the presumptive
diagnosis of UTI. Markers for infection in a urinalysis are the presence of
nitrites, leukocytes esterase, bacteria, or white blood cells.16 An
infant who is evaluated for fever may require lumbar puncture, in addition to
blood cultures, to evaluate UTI with secondary bacteremia.
Diagnosis
The diagnoses for acute
pyelonephritis, cystitis, and asymptomatic bacteriuria are made by the
presence of bacteria in the urine, usually based on a clean midstream urine
sample. There must be a minimum of 105 colony-forming units per
milliliter (cfu/mL) of uropathogens for diagnosis of acute pyelonephritis and
asymptomatic bacteriuria but only 103 cfu/mL for the diagnosis of
cystitis. Up to one third of cystitis cases would be missed if the criterion
for diagnosis were the same as that for upper tract infections.17
Uncomplicated UTI refers to cystitis and
pyelonephritis that occurs in young, healthy, nonpregnant, or ambulatory
postmenopausal women--all of whom have an anatomically and functionally normal
urinary tract. Patients with complicated UTIs are those who have an associated
risk for infection in the urinary tract (e.g., those with neurogenic bladder,
nephrolithiasis, hospital-acquired infection, diabetes, indwelling catheters,
or who are immunosuppressed). Resistant organisms can be seen in either
complicated or uncomplicated UTIs.17
Because UTIs occur in young healthy men
infrequently, there is debate whether to consider these infections complicated
or uncomplicated, given the paucity of studies in this patient group. The
organisms and sensitivity patterns seem to be the same as in women with
uncomplicated cystitis. Empiric treatment for cystitis would utilize the same
antibiotic choices used in women, but three-day regimens are not recommended
because of the lack of supporting data. A pretreatment urine culture should be
obtained in all men with UTI; however, a posttreatment culture is usually not
necessary. Recurrent infections require evaluation for prostatitis, and if
negative, an evaluation for anatomic abnormalities should ensue.17
In practicality, using bacteriuria for the
diagnosis of cystitis can be cumbersome, since urine culture is not performed
in many cases of uncomplicated cystitis. Urine microscopy has a low
sensitivity (40% to 70%) but a high specificity (85% to 95%) for the diagnosis
of UTI. Pyuria is present in most cases of pyelonephritis--estimated to be
about 90%. Presence of pyuria increases the sensitivity (95%) and specifity
(71%) for the diagnosis of acute pyelonephritis. White cell casts always point
to an upper tract infection.18 Urine culture is positive in 90% of
cases of pyelonephritis, and 20% of hospitalized cases have positive blood
cultures. The cultures were obtained prior to antibiotic therapy in these
cases. There is no evidence that positive blood cultures indicate a more
complicated course in immunocompetent individuals.19
Dipstick urinalysis has become the most
frequently used test due to its cost and fast results. Studies have shown that
dipstick urinalysis, in combination with clinician judgment, greatly improves
diagnostic accuracy in the patient with nonspecific symptoms. Urine dipstick
is positive if there is a presence of nitrate and/or if there is a positive
reaction greater than or equal to trace leukocyte esterase.20
Patients should be screened for asymptomatic
bacteriuria in cases of pregnancy or prior to a urologic procedure or surgery.
Urine culture is the method of choice, since the other types of studies lack
the sensitivity and specificity in these cases. During pregnancy, UTIs are
common in the sixth week and peak during weeks 22 to 24. Pregnant women have a
prevalence of asymptomatic bacteriuria of 2% to 7%. If untreated, 20% to 30%
of these women will develop acute pyelonephritis later in pregnancy. When
pyelonephritis occurs late in pregnancy, there is an association with preterm
labor. Treatment has been shown to decrease this risk by 90%. The current
recommendation by the Infectious Diseases Society of America is to obtain a
urine culture at the end of the first trimester, and if positive, to treat the
bacteriuria. Intrauterine growth retardation and neonatal death have also been
linked to asymptomatic bacteriuria. There are no indications for screening
elderly patients in the community or in institutions for asymptomatic
bacteriuria, as treatment has not been shown to benefit these individuals.
21
Imaging
The diagnosis of pyelonephritis
can usually be made by history, physical examination, and laboratory tests.
Imaging may be necessary when the diagnosis is in question, when there are
recurrent infections, or if the patient responds poorly to appropriate
antibiotic therapy after three days. Computed tomography (CT) with intravenous
(IV) contrast is the test of choice when evaluating the urinary tract. The
most common CT finding in pyelonephritis is wedge-shaped lesions of decreased
attenuation with or without swelling. Anatomic abnormalities and perinephric
abscesses can also be seen on contrast-enhanced scans. Renal ultrasound is
also used to evaluate the collecting system and pyelonephritis and may show
ureteral dilation, suggesting obstruction. Although renal ultrasound is
helpful, a CT scan is more sensitive. Magnetic resonance imaging may be used
in patients who are allergic to iodinated contrast.22
Diagnostic studies for UTI in pediatric
patients are important, given the potential long-term sequelae associated with
undiagnosed or recurrent UTIs. Recurrence may be a marker for genitourinary
abnormalities, and imaging is recommended after the first infection with
concomitant fever. Vesicoureteral reflux encompasses a variety of conditions
that represent the most common abnormality observed in infants and young
children. This condition can lead to recurrent UTIs, resulting in secondary
scarring that causes an increased risk of progression to renal disease into
adulthood. Children with poor clinical response to appropriate treatment
should have immediate renal and bladder ultrasound (RBUS) and voiding
cystourethrography (VCUG).23
Treatment
Prevention of UTI is always the
goal of clinicians. There are many proven and unproven strategies to
accomplish this task (see table 1). Most UTIs will clear spontaneously if
untreated, but symptoms may persist for a significantly longer period of time.
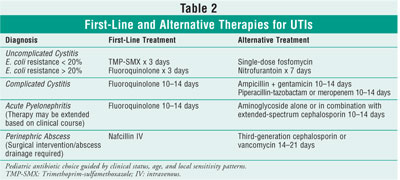
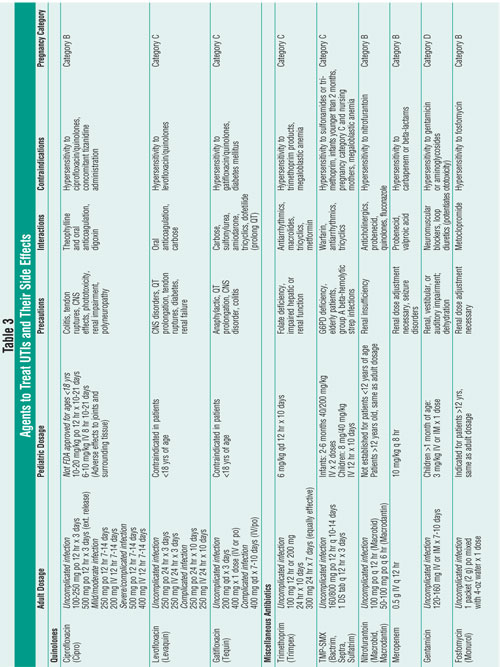
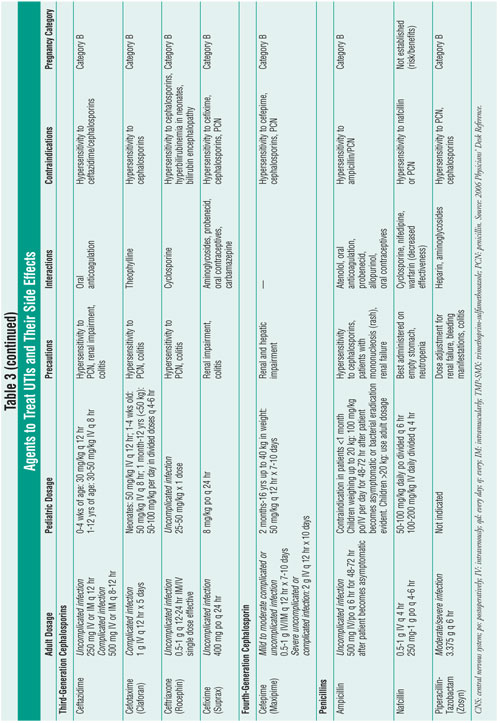
Tables 2 and 3 review first-line and alternative treatments for UTI, outlining
the adverse effects of each agent.

In one study, one in 38 women progressed from
uncomplicated cystitis to pyelonephritis without treatment.17 The
most common organisms by far are gram-negative bacilli. Escherichia coli
causes 80% of all community-acquired UTIs among otherwise healthy
individuals, and roughly half of UTIs occur in hospitalized patients and
diabetic patients.2 Other bacteriuria include gram-negative rods
such as Proteus, Klebsiella, and Enterobacter. Antibiotic
resistance has become an important factor to consider in the treatment of
infections. Resistance may occur in ambulatory, institutionalized, and
hospitalized infections. E. coli resistance has been progressing for
more than a decade.24
E. coli Resistance: The resistance
pattern is not uniform in the U.S.; in 2000, it ranged from 10% in the
Northeast to 22% in the West.25 The resistance to
trimethoprim-sulfamethoxazole (TMP-SMX) has been significant, and therefore,
it is no longer the first therapeutic choice in complicated UTI or
pyelonephritis. Given the concern of resistance progressing with the
fluoroquinolones, TMP-SMX remains first-line therapy for the most common
uncomplicated cystitis.24 Fluoroquinolones are the primary therapy
for complicated cystitis and acute pyelonephritis. There is still relatively
low resistance to fluoroquinolones for E. coli, but resistance is
significantly emerging with enterococci. Tendonitis and, rarely, Achilles heel
rupture in the geriatric population have been noted with the quinolones.26
Nitrofurantoin is associated with low levels of E. coli resistance,
which is theorized to be due to its multiple mechanisms of action, but the
agent has poor tissue and plasma concentration. Thus, nitrofurantoin has no
use beyond the urinary tract.24 Beta-lactams have significant
problems with resistance--up to 40% in 2002. Fosfomycin is indicated only for
uncomplicated cystitis and has low reported resistance.24
Urinary Catheters:
Any catheterization of the bladder increases the risk of infection, but
indwelling has a higher risk than intermittent catheterization. Treatment of
asymptomatic bacteriuria in patients with chronic indwelling catheters has
shown no benefit.21 When a patient becomes symptomatic, blood and
urine cultures should be obtained prior to treatment. Antibiotics should be
initiated immediately after cultures and should be tailored to the patient,
institution, and susceptibility patterns.
Cystitis:
For uncomplicated cystitis in women, a three-day course of TMP-SMX is
recommended; if the patient is older, a longer course of therapy (seven to 10
days) should be considered. This recommendation applies to areas where E. coli
resistance is less than 20%. Studies have examined three-day courses of
ciprofloxacin versus TMP-SMX, and the results of bacteria eradication were
similar in uncomplicated cystitis. If the patient is allergic to sulfa,
alternative therapies are nitrofurantoin for seven days or single-dose
fosfomycin. Fluoroquinolones are first-line therapy in areas where E. coli
resistance is greater than 20%.27 In pregnant patients with
cystitis, a seven-day duration is preferred for nitrofurantoin or cephalexin.
However, fosfomycin in a single dose is another alternative.27
Women with recurrent bouts of cystitis may need to consider an alternative
form of contraception. Spermicides and diaphragms may predispose women to
infection. Postcoital prophylaxis should be instituted only after the current
infection is treated and a negative urine culture is obtained. Single-dose
TMP-SMX, nitrofurantoin, or fluoroquinolones can be used after intercourse.
18
Complicated Cystitis:
In complicated UTIs, a wide range of bacteria can cause infection.
Fluoroquinolones are effective first-line treatment, given their broad
spectrum of coverage for mild to moderate infections. For more seriously ill,
hospitalized patients, initial empiric therapy includes ampicillin and
gentamicin. Alternative choices of antimicrobials are third-generation
cephalosporins, pipercillin-tazobactam, meropenem, or ciprofloxacin, based on
sensitivities. The expected length of treatment for complicated UTIs is 10 to
14 days. Switching from IV to postoperative coverage largely depends on
clinical improvement. Pseudomonas and Enterococcus are difficult
to treat and may require a longer course of IV antibiotics. Repeat cultures
are recommended one to two weeks after the completion of therapy. If a patient
fails to respond to treatment, repeat cultures should be attained along with
imaging studies to rule out persistent infection secondary to anatomic
abnormalities or stone disease.28
Most complicated UTIs are nosocomial in
origin. The most common pathogens are gram-negative bacteria, which have been
shown to colonize the meatus. Hand washing and good aseptic technique
are imperative in preventing infection. Practitioners must be cautious when
determining the need for indwelling Foley catheters. Intermittent
catheterization reduces the risk of bacteriuria. If a chronic indwelling Foley
catheter is indicated, then the catheter should be changed at least once every
30 days.
Perinephric abscess is considered a
complicated UTI. The most common causative organism is Staphylococcus aureus
. First-line treatment is IV nafcillin or a third-generation cephalosporin.
Vancomycin may be used as an alternative. In most cases, patients will need
surgical intervention or aspiration of the infected fluid collection.29
Acute Pyelonephritis:
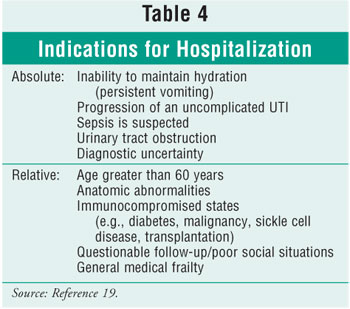
Many patients with pyelonephritis need to be hospitalized. (See Table 4 for
indications.) Patients who do not meet absolute indications for
hospitalization are treated successfully in about 90% of cases with outpatient
oral regimens. After the urine culture is obtained, empiric therapy consists
of an oral fluoroquinolone for outpatient treatment. Patients requiring
hospitalization should receive an IV fluoroquinolone or an aminoglycoside
alone or in combination with an extended-spectrum cephalosporin or ampicillin.
Oral treatment may be instituted once the patient is afebrile, clinically
improved, and able to tolerate oral medications and hydration.19
The duration of therapy in pyelonephritis is controversial. In acute,
uncomplicated pyelonephritis, therapy duration ranges from seven to 14 days.
Beta-lactams generally require 10 to 14 days, while the more common
fluoroquinolones or TMP-SMX can require a duration of seven to 10 days.
Complicated pyelonephritis may require a longer course of treatment,
especially if the patients are immunocompromised. Generally, these patients
require 14 to 21 days of therapy or even longer depending on their clinical
course.17
Pediatric Population
Group B streptococcus is the most
common organism seen in neonates. Overall, E. coli is the most typical
organism found in the pediatric population, accounting for 75% to 90% of all
cases. Hospitalized children may require frequent catheterizations, making
Enterococcus and Pseudomonas a possible consideration.30
Management of UTI in the neonatal population
is largely geared toward preventing bacteremia, given the high risk of
meningitis in this age group. Another goal in the treatment of pediatric
patients is prompt diagnosis and empiric treatment to prevent progression of
renal disease and to identify any urinary tract abnormalities.15
Infants and children younger than 5 years of age should be hospitalized if the
clinical suspicion is high for systemic infection. According to the American
Academy of Pediatrics (AAP), hospitalization should also be considered if
noncompliance is a concern, if outpatient treatment has failed, or if the
patient is immunocompromised. A hospitalized patient who remains afebrile for
48 hours and tolerates oral antibiotics may be discharged with continued
therapy on oral antibiotics. Once the infection has cleared, additional tests
may be required to rule out abnormalities of the urinary tract (RBUS and VCUG).
23
The choice of antimicrobial depends on a
number of different factors, such as the patient's clinical status, age (which
may be associated with specific pathogens), and local sensitivity patterns in
the area of practice.15 For neonates with a diagnosis of UTI and
hydronephrosis, amoxicillin is recommended at one third of the normal dosage.
31 Sulfamethoxazole, TMP-SMX, or cephalosporins are beneficial
first-line choices for children who are able to tolerate oral medications.
32 In children who have failed oral therapy, ceftriaxone or gentamicin
administered intramuscularly has been shown to be effective in treating UTI.
23 The AAP recommends seven to 10 days of treatment for uncomplicated
UTIs. For febrile infants with a UTI and children with presumed
pyelonephritis, a 14-day course of therapy is recommended.
Prophylactic antimicrobials may be used in
children with symptomatic UTIs with anatomic abnormalities. There are few data
to support the use of antibiotics in children without anatomic anomalies.
33
References
1. Centers for Disease Control
and Prevention. Guideline for the Prevention of catheter-associated Urinary
Tract Infections. Available at: www.cdc.gov/ncidod/dhqp/gl_catheter_assoc.html.
2. Brown P, Foxman B. Epidemiology of
urinary tract infections transmission and risk factors, incidence and costs.
Infec Dis Clin N Am. 2003;17: 227-241.
3. IDSA Practice Guidelines for
antimicrobial treatment of uncomplicated acute bacterial cystitis and acute
pyelonephritis in women. Available at: www.uptodateonline.com.
4. Stamm WE, Norrby SR. Urinary tract
infections: Disease panorama and challenges. J Infec Dis. 2001;183:S1.
5. Green MB, Bailey PP. Infectious
processes: Urinary tract infections and sexually transmitted diseases. In:
Buttaro TM, Trybulski J, Bailey PP, Sandberg-Cook J, eds. Primary Care: A
Collaborative Practice. St. Louis, MO: Mosby; 2003.
6. Foxman B. Epidemiology of urinary
tract infections: incidence, morbidity and economic costs. Am J Med.
2002;113:5-13.
7. NCHS: Ambulatory care visits to
physician offices, hospital outpatient departments, and emergency departments:
United States, 1997. Vital and Health Statistics Series 1999:13(143).
8. Warren, JW, Steinberg, L, Hebel,
JR, et al. The prevalence of urinary catheterization in Maryland nursing
homes. Arch Intern Med. 1989:149:1535.
9. Wettergren B, Jodal U, Jonasson G.
Epidemiology of bacteriuria during the first year of life. Acta Paediatr
Scand. 1995;74:925.
10. To T, Agha M, Dick PT, Feldman W.
Cohort sudy on the circumcision of newborn boys and subsequent risk of urinary
tract infection. Lancet. 1998;352:1813-1816.
11. Nuutinen M, Uhari M. Recurrence
and follow-up after urinary tract infection under the age of 1 year.
Pediatric Nephrology. 2001;16:69-72.
12. Hooten TM, Scholes D, Hughes JP,
et al. A prospective study of risk factors from symptomatic urinary tract
infections in young women. N Engl J. Med. 1996;335:468-474.
13. Ronald A, Ludwig E. Urinary tract
infections in adults with diabetes. Int J Antimicrobial Agents.
2001;17:287-292.
14. MacNeily AE. Pediatric urinary
tract infections: current controversies. Canadian J Urol. 2001;8:18-23.
15. Schlager TA. Urinary tract
infections in infants and children. Infec Dis Clin N Am.
2003;17:353-365.
16. Layton KL. Diagnosis and
management of pediatric urinary tract infections. Clin Fam Practice.
2003;5:367-382.
17. Hooten T. The current management
strategies for community-acquired urinary tract infection. Infect Dis Clin
N Am. 2003;17:303-332.
18. Fihn S. Acute uncomplicated
urinary tract infection in women. N Engl J Med. 2003;349:259-266.
19. Ramakrishnan K, Scheid D.
Diagnosis and management of acute pyelonephritis in adults. Am Fam Physician
. 2005;71:933-942.
20. Sultana R, Zalstein S, Cameron P,
Campbell D. Dipstick urinalysis and the accuracy of the clinical diagnosis of
urinary tract infection. J Emerg Med. 2001;20:13-19.
21. Nicolle L. Asymptomatic
bacteriuria: When to screen and when to treat. Infec Dis Clin N Am.
2003;17:367-394.
22. Kawashima A, Leroy A. Radiologic
evaluation of patients with renal infections. Infec Dis Clin N Am.
2003;17:433-456.
23. Shortliffe L, McCue J. Urinary
tract infection at age extremes: pediatrics and geriatrics. Am J Med.
2002;133:55-65.
24. Gupta K. Emerging antibiotic
resistance in urinary tract pathogens. Infec Dis Clin N Am.
2003;17:243-259.
25. Gupta K, Sahm DF, et al.
Antimicrobial resistance among uropathogens that cause community acquired
urinary tract infections in women: a nationwide analysis. Clin Infec Dis
. 2001;33:89.
26. Gold L, Igra H.
Levofloxacin-induced tendon rupture: A case report and review of the
literature. J Am Board Fam Practice 2003;16:458-460.
27. Mehnert-Kay S. Diagnosis and
management of uncomplicated urinary tract infections. Am Fam Physician.
2005;72:451-456.
28. Stamm WE, Hooton TM. Management
of urinary tract infections in adults. N Engl J Med. 1993;329:1328-1334.
29. Sanford Guide for Microbiology;
Perinephric Abscess, 2005:22-23.
30. Elder JS. Urinary tract
infections. In: Behrman RE, Kliegman FM, Jenson HB, eds. Nelson Textbook of
Pediatrics. Philadelphia, PA: W.B. Saunders; 2000:1624-1625.
31. Caldemone A. Antibiotic
prophylaxis for infants with congenital hydronephrosis [letter]. Pediatric
Infec Dis J. 1999;18:398-399.
32. American Academy of Pediatrics.
Subcommittee on Urinary Tract Infection. Practice parameter: diagnosis,
treatment, and evaluation of urinary tract infection in infants and young
children. Pediatrics. 1999;103:843-852.
33. Dagan R, Phillip M, Watemberg NM,
et al. Outpatient treatment of serious community-acquired pediatric infections
using once daily intramuscular ceftriaxone. Pediatric Infec Dis J.
1987;6:1080-1084.
34. UrologyHealthChannel. Available:
www.urologychannel.com/uti/alternativetreatment.shtml.
35. Lynch D. Cranberry for prevention
of urinary tract infections. Am Fam Physician. 2004;70:2175-2177.
To comment on this article, contact
editor@uspharmacist.com.






