US Pharm. 2023;48(7):26-32.
ABSTRACT: Patients who have undergone organ transplant surgery require immunosuppressive therapy to decrease and/or prevent rejection episodes. The therapy regimens often involve multiple medications, which require routine monitoring and adjustments when warranted. Adherence to these therapies is critical to successful clinical outcomes. As integral healthcare team members, pharmacists are well poised to educate transplant patients about the drug regimens generally prescribed post transplant, including immunosuppressive agents, proper use, and potential adverse effects. Pharmacists can also be instrumental in making clinical recommendations to optimize drug efficacy and safety and to manage and diminish the incidence of adverse effects while improving clinical outcomes and health-related quality of life.
For numerous individuals with end-stage organ failure, organ transplant surgeries (e.g., kidney, liver, heart, lungs, and pancreas) have given these patients an opportunity to save their lives and prolong their survival. Over the past 60 years, organ transplants have been considered one of the most noteworthy advances in medicine.1,2 According to preliminary data from the United Network for Organ Sharing (UNOS), there were multiple milestones for transplants in 2022.1 The organization reported that in 2022, there were more than 42,887 organ transplants performed in the United States, marking a rise of 3.7% compared with those performed in 2021.1 Additionally, UNOS states that 2022 marked the first year on record in which 24,498 kidney transplants were performed in the United States, with a 3.4% increase over those performed in 2021. In addition, annual records were documented for liver (9,528), heart (4,111), and lung (2,692) transplants.1
Patients and their families often face numerous physical, psychological, and financial challenges throughout the transplantation process, including adapting to a complex regimen of various medications to decrease the incidence of organ rejection, infections, and mortality. Therefore, implementing a multidisciplinary approach to patient education by providing information about the transplant process, available resources, and support services is essential in assisting patients and family members to understand expectations and adjust to life after the transplant.
In general, posttransplant patients are often discharged from the hospital with complex drug regimens that involve multiple medications, including lifelong use of immunosuppressant agents that are prescribed to decrease and/or thwart the incidence of acute or chronic organ rejection as well as medicines to manage other comorbidities and to reduce and/or prevent certain infections.2 Pharmacists are essential members of the multidisciplinary healthcare team for posttransplant patients and have multifaceted roles such as drug monitoring, medication reconciliation, patient counseling, and making clinical recommendations to optimize drug safety and efficacy and minimize adverse effects.2
Various publications reveal that post transplant, some patients may take as many as five to 10 medications or more.2-5 Regimens may also require patients to take multiple medications one to four times daily. These complex multidrug regimens are often associated with an increased risk of drug-drug interactions, leading to an augmented incidence of adverse effects and risk of nonadherence.2-5 The National Kidney Foundation indicates that due to the complexity of drug regimens, an estimated 20% to 60% of transplant patients have revealed that they may miss a medication dose or that they are noncompliant with medication.5
It is well documented in the literature that noncompliance with posttransplant drug therapy is correlated with a host of complications, including acute and chronic rejection.2-5 Other research indicates that nonadherence during the pretransplant phase is often a factor that may aid clinicians in predicting if a patient will be nonadherent in the posttransplant phase, and it is imperative to identify and address these issues to improve clinical outcomes.6-9 Additionally, nonadherence with immunosuppressive medications is a major risk factor for graft loss, morbidity, and mortality.6-9
Classification of Immunosuppressive Agents
Posttransplant immunosuppressive agents are prescribed for early-stage immunosuppression, the management of late-stage immunosuppression, or the maintenance of organ rejection; therefore, in general they are classified as induction therapies, maintenance therapies, and antirejection therapies.10-12 Posttransplant immunosuppression classically incorporates a combination of medications based on individualized patient factors, medical and medication history, and the type of organ transplant.12,13
According to UNOS:
• Induction therapies are therapies administered immediately after transplant in intensified doses to thwart episodes of acute rejection. While these therapies may be continued post hospital discharge for at least 30 days after transplant, these therapies are not prescribed for long-term immunosuppressive maintenance.12
• Maintenance therapies consist of all immunosuppressive medications prescribed before, during, or after transplant and with the purpose of long-term use. Additionally, maintenance immunosuppression does not involve any immunosuppressive medications administered to manage rejection episodes or induction.12
• Antirejection immunosuppression involves all immunosuppressive medications prescribed for dealing with an episode of acute rejection in the initial posttransplant period or during a specific follow-up period, typically up to 30 days after the diagnosis of acute rejection.12
According to the recently published consensus recommendations for the usage of maintenance immunosuppression in solid organ transplantation endorsed by the American College of Clinical Pharmacy, American Society of Transplantation, and the International Society for Heart and Lung Transplantation, tremendous progress has been made with regard to maintenance immunosuppressive therapies (M-IMS), which in turn have enhanced clinical outcomes in those who have undergone solid organ transplantation over the past 30 years.13 The panel of experts for the consensus recommendations states, “Uninterrupted access to immunosuppression is paramount to minimize rejection and maintain allograft and patient survival.”13 The consensus panel experts also state, “The need for lifelong maintenance immunosuppression (M-IMS) is nearly universal as the risk of rejection is omnipresent. Nonadherence to M-IMS is a contributing cause of poor post-transplant outcomes, with barriers to medication access a leading risk factor for nonadherence.”13
The panel notes that these recommendations are aimed at offering transplant clinicians a synopsis of the most current literature on M-IMS therapies and supporting transplant team members who assist transplant patients in accessing these lifesaving medications.13 The panel of experts also indicates that there is no universal approach to M-IMS management, and selected agents vary from institution to institution and are often contingent on numerous factors, including transplanted organ, center-specific protocol, provider expertise, insurance formularies, recipient characteristics and tolerability, and patient insurance.13
Studies have indicated that posttransplant patients often deal with physical and psychological issues as well as adjusting to day-to-day life and productivity, which may be linked to the complexity of drug regimens that involve multiple medications, managing the timing of doses, and the increased risk of developing adverse effects.14,15 Findings from a cross-sectional, qualitative study exploring self-management challenges and wishes for patient support in transplant recipients revealed that they wanted to receive more patient education, be able to share experiences with other transplant patients, and talk about not only medical issues but also emotional and social problems with their healthcare providers; they also needed positive feedback from their healthcare providers.15 Finally, the authors indicated that the “one size fits all” educational approach was not beneficial and that care should be tailored to patient need and level of understanding.15
Studies reveal that as many as 70% of posttransplant patients do not adhere to their medication regimens as directed for various reasons, including the inability to afford medications due to high medication costs and lack of proper patient education that resulted in a deficit of understanding of how and when to take medications correctly due to multiple medication regimens.16
Posttransplant Immunosuppressants
Post transplant, patients may be prescribed immunosuppressive agents and anti-infectives. The anti-infectives include an antimicrobial prophylaxis, which may consist of one or more of the following: antibacterials, antifungals, and antivirals to prevent infections, especially during the first month post transplant since patients are at greater risk of developing opportunistic infections such as Candida esophagitis, cytomegalovirus, herpes simplex virus, and Pneumocystis jirovecii.17-19 It is essential that clinicians routinely monitor patients since some antimicrobials can affect and/or interfere with the metabolism of immunosuppressive agents by augmenting or reducing serum levels of immunosuppressive agents that may heighten an individual’s risk of severe adverse effects.18 Additionally, patients may be prescribed medications for the treatment of other comorbidities and medications to address the adverse drug reactions associated with immunosuppressive agents, such as antiemetics for nausea and vomiting and/or medications for sleep issues, depression, and anxiety.17,18 Some transplant clinicians may also recommend the use of nutritional supplements tailored to patient need.
Studies reveal that long-term use of immunosuppressive agents is often correlated with the onset of posttransplant diabetes, hypertension, hyperlipidemia, cardiovascular disease, and infections. This, in turn, augments mortality rates, therefore highlighting the significance of tailoring therapy to patient need to optimize drug safety, minimize adverse effects, and improve clinical outcomes.20,21 Different studies indicate a range of 10% to 40% incidence of posttransplant diabetes.20,21
Multiple publications indicate that the major M-IMS agents that are typically prescribed include a combination of agents, including calcineurin inhibitors (tacrolimus and cyclosporine), antimetabolites (mycophenolate mofetil and azathioprine), mammalian target of rapamycin inhibitors (everolimus and sirolimus), and corticosteroids.10-14,21 Transplant experts note that the primary goal in posttransplant patients is to individualize therapy to include the optimal combination of immunosuppressive agents, which can improve patient survival by preventing acute rejection while also preventing or decreasing the incidence of adverse effects.10-14,21
The 2019 Organ Procurement and Transplantation Network Annual Data Report revealed that the most frequently prescribed posttransplant M-IMS regimen included tacrolimus, mycophenolate mofetil (MMF), and corticosteroids for kidney (65%), pancreas (67%), liver (65%), heart (86%), and lung (80%) transplant recipients.13 See TABLE 1 for additional information on immunosuppressive agents used in solid-organ transplantation.
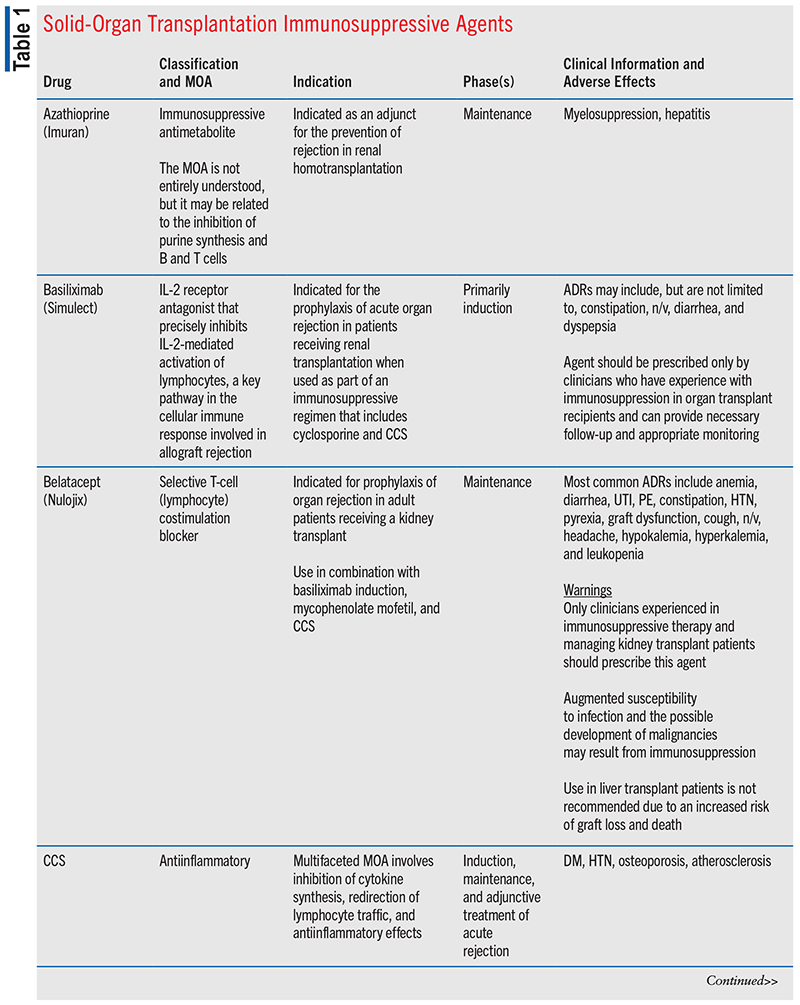

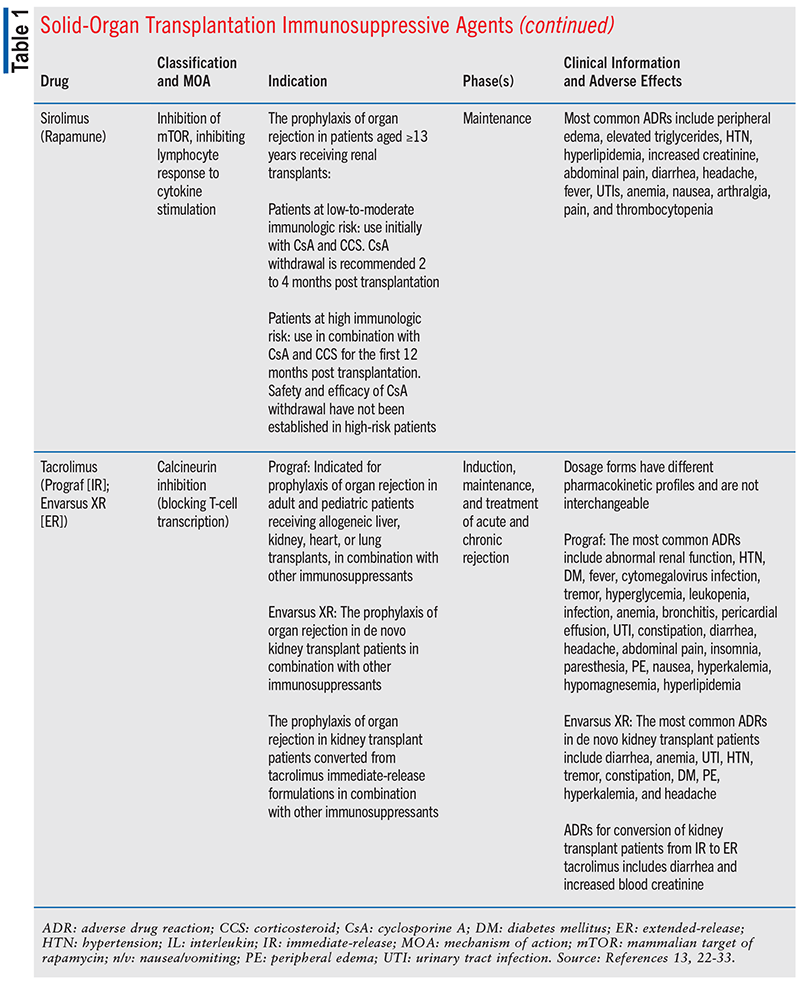
According to the consensus recommendations mentioned above, the most frequently prescribed corticosteroids for the prevention and treatment of organ rejection are IV methylprednisolone and oral prednisone, and a large percentage of transplant recipients are continued on corticosteroids indefinitely.13 The panel of experts also indicates that early corticosteroid discontinuation post transplant is a goal due to the considerable morbidity risk correlated with chronic and long-term use of these agents.13
Identifying and Addressing Nonadherence
Pharmacists can be instrumental in assisting patients in the management of complex posttransplant regimens and make clinical recommendations to improve adherence when possible. Various publications indicate that nonadherence is common among posttransplant patients and may be classified as intentional (conscious refusal to take prescribed medication) or unintentional (nondeliberate, such as missing medication doses), and contributing factors may be patient-related, therapy-related, disease-related, socioeconomic, and/or healthcare-related.22-24
According to a study in the journal Nephrology, nonadherence to immunosuppressant medications augments the likelihood of allograft rejection and loss.22 In this study involving a cohort of 161 adult renal transplant patients, researchers sought to explore the incidence of nonadherence and barriers to adherence with immunosuppressant medications.22 The results revealed that 55% were classified as nonadherent, with 45% delaying doses and 25% missing doses. The authors indicated that nonadherent patients were more prone to not remembering doses and more prone to skip doses when their daily routine changed or when the cost of medication was a factor. Additionally, nonadherent patients had less self-reported knowledge than adherent patients; over 50% of patients self-reported nonadherence, with the primary factors contributing to nonadherence being forgetfulness and skipped doses. The authors recommended that interventions tailored to patient need and level of understanding may enhance adherence.22
Another study indicated that among adults greater than 12 months post kidney transplant, nonadherence with immunosuppressive therapy was recorded as 33.6% and was correlated with negative impacts on mental health–related quality of life and anxiety and depression.23,24 Studies also noted that adverse effects associated with immunosuppressive therapy may affect patient adherence.23,24
A systematic review published in Clinical Kidney Journal indicated that nonadherence to medication rates range from 2.3% to 72.2% among lung transplant recipients.25
In a systematic review published in Transplantation Reviews, nonadherence to immunosuppressive therapy among heart transplant patients may correlate with an increased risk of transplant coronary artery disease and acute late rejection and mortality.26 The authors noted that simplified drug regimens, including utilizing once-daily dosing immunosuppressives such as tacrolimus, medication reminders, and mobile app reminders, were related to greater adherence.26
According to another publication in Clinical Kidney Journal, authors recommended strategies that may aid in thwarting and addressing nonadherence; they include collaborative efforts between healthcare providers involved in patient care, including interventions from pharmacists, electronic reminders such as medication reminders via phone alarms and applications, simplifying drug regimens when possible such as once-daily or twice-daily regimens, and identifying patient risk factors via multidisciplinary measures from the healthcare team.27
The Role of the Pharmacist
An abundance of clinical evidence indicates that pharmacists can enhance outcomes in transplant patients through clinical recommendations and patient education initiatives.28 Additionally, a study published in the International Journal of Clinical Pharmacy indicates that pharmacists can be a valuable resource and improve pharmaceutical care and optimize medication safety, especially since transplant patients often require complex medication regimens and medication changes, augmenting the risk for drug/drug interactions and adverse effects.29 Patient education and adherence to therapy are critical components to attaining successful clinical outcomes. Patients should be encouraged to take an active part in their care, which may improve adherence and overall health-related quality of life.
Pharmacist Roles in the Management of Transplant Patients
• Making clinical recommendations and monitoring potential drug-drug interactions and contraindications, as well as patient response to complex posttransplant medication regimens
• Recommending treatment regimens based on efficacy and safety data tailored to patient needs
• Implementing patient education initiatives to enhance patient adherence and improve clinical outcomes
• Conducting ongoing clinical monitoring and making changes when warranted to optimize medication efficacy and safety
• Identifying and addressing adverse effects when warranted
• Educating patients on the drugs prescribed, dosage, administration, indication for use, monitoring parameters, and potential adverse effects
• Instructing patients on what to do in case of a missed dose
• Providing information on the handling and storage of medication
• Recommending measures such as medication reminders to ensure doses are not missed
• Providing tips on staying organized, such as using pill boxes with labels and refill reminders, to ensure that medications are filled on time.
Pharmacists can also suggest that patients obtain a MedicAlert ID tag or bracelet indicating that they are transplant recipients and continue to maintain a current list of all medicines, including nonprescription medications. Patients should also be instructed to always discuss using any new medications, including OTC products and supplements, with their transplant care provider prior to using them.
Conclusion
The transplant process is a life-changing experience for both patients and their families, especially during the posttransplant period. Empowering patients with knowledge, encouragement, and support is essential as patients adjust to life posttransplant and to improve health-related quality of life.
REFERENCES
1. United Network for Organ Sharing. 2022 organ transplants again set annual records. January 10, 2023. https://unos.org/news/2022-organ-transplants-again-set-annual-records/. Accessed June 12, 2023.2. Sam S, Guérin A, Rieutord A, et al. Roles and impacts of the transplant pharmacist: a systematic review. Can J Hosp Pharm. 2018;71(5):324-337.
3. Denhaerynck K, Dobbels F, Cleemput I, et al. Prevalence, consequences, and determinants of nonadherence in adult renal transplant patients: a literature review. Transpl Int. 2005;18(10):1121-1133.4. Pinsky BW, Takemoto SK, Lentine KL, et al. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant. 2009;9(11):2597-2606.
5. Anderson-Haag T. Transplant medications: forget me not! National Kidney Foundation. www.kidney.org/transplantation/transaction/TC/summer09/TCsm09_ForgetMeNot. Accessed June 12, 2023.6. Sandal S, Chen T, Cantarovich M. Evaluation of transplant candidates with a history of nonadherence: an opinion piece. Can J Kidney Health Dis. 2021;8:1-7.
7. Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the AASLD and the American Society of Transplantation. Hepatology. 2014;59:1144-1165.8. EASL clinical practice guidelines: liver transplantation. J Hepatol. 2016;64(2):433-485.
9. Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant. 2016;35(1):1-23.10. Pellegrino B, Schmidt RJ, Onder S, et al. Immunopsupression. Medscape. March 1, 2021. https://emedicine.medscape.com/article/432316-overview#a3. Accessed April 24, 2023.
11. Hussain Y, Khan H. Immunosuppressive drugs. Encyclop Infect Immun. 2022:726-740.12. United Network for Organ Sharing. Types of immunosuppressants. www.transplantliving.org/after-the-transplant/preventing-rejection/types-of-immunosuppressants/. Accessed June 12, 2023.
13. Nelson J, Alvey N, Bowman L, et al. Consensus recommendations for the use of maintenance immunosuppression in solid organ transplantation: endorsed by the American College of Clinical Pharmacy, American Society of Transplantation, and the International Society for Heart and Lung Transplantation. Pharmacotherapy. 2022;42(8):599-633.14. Yang FC, Chen HM, Huang CM, et al. The difficulties and needs of organ transplant recipients during postoperative care at home: a systematic review. Int J Environ Res Public Health. 2020;17(16):5798.
15. Been-Dahmen JMJ, Grijpma JW, Ista E, et al. Self-management challenges and support needs among kidney transplant recipients: a qualitative study. J Adv Nurs. 2018;74(10):2393-2405.16. Tschida S, Aslam S, Khan TT, et al. Managing specialty medication services through a specialty pharmacy program: the case of oral renal transplant immunosuppressant medications. J Managed Care Pharm. 2013;19(1):26-41.
17. de la Vega A, Luong D. Post-transplant patient education and medication adherence. Texas Kidney Foundation. April 28, 2017. https://txkidney.org/wp-content/uploads/2017/03/Post-Transplant-Patient-Education-and-Compliance-v.pdf?x15235. Accessed June 12, 2023.18. Bhagat V, Pandit RA, Ambapurkar S, et al. Drug interactions between antimicrobial and immunosuppressive agents in solid organ transplant recipients. Indian J Crit Care Med. 2021;25(1):67-76.
19. Kumar R, Ison MG. Opportunistic infections in transplant patients. Infect Dis Clin North Am. 2019;33(4):1143-1157.20. Ahmed SH, Biddle K, Augustine T, et al. Post-transplantation diabetes mellitus. Diabetes Ther. 2020;11:779-801.
21. Parlakpinar H, Gunata M. Transplantation and immunosuppression: a review of novel transplant-related immunosuppressant drugs. Immunopharmacol Immunotoxicol. 2021;43(6):651-665.22. Cossart AR, Staatz CE, Campbell SB, et al. Investigating barriers to immunosuppressant medication adherence in renal transplant patients. Nephrology. 2019;24(1):102-110.
23. Covvey JR, Mancl EE. Pharmaceutical care in transplantation: current challenges and future opportunities. Nanomedicine. 2019;14(20):2651-2658.24. Scheel JF, Schieber K, Reber S, et al. Psychosocial variables associated with immunosuppressive medication non-adherence after renal transplantation. Front Psychiatry. 2018;9:23.
25. Hu L, Lingler JH, Sereika SM, et al. Nonadherence to the medical regimen after lung transplantation: a systematic review. Heart Lung. 2017;46(3):178-186.26. Hussain T, Nassetta K, O’Dwyer LC, et al. Adherence to immunosuppression in adult heart transplant recipients: a systematic review. Transplant Rev. 2021;35(4):100651.
27. Gandolfini I, Palmisano A, Fiaccadori E, et al. Detecting, preventing and treating non-adherence to immunosuppression after kidney transplantation. Clin Kidney J. 2022;15(7):1253-1274.28. Sam S, Guérin A, Rieutord A, et al. Roles and impacts of the transplant pharmacist: a systematic review. Can J Hosp Pharm. 2018;71(5):324-337.
29. Mulder MB, Doga B, Borgsteede SD, et al. Evaluation of medication-related problems in liver transplant recipients with and without an outpatient medication consultation by a clinical pharmacist: a cohort study. Int J Clin Pharm. 2022;44(5):1114-1122.30. Hertl M. Overview of transplantation. Merck Manual Professional Version. September 2022. https://www.merckmanuals.com/professional/immunology-allergic-disorders/transplantation/overview-of-transplantation?query=transplant. Accessed June 12, 2023.
31. Imuran (azathioprine) package insert. Hunt Valley, MD: Prometheus Labs Inc; May 2011.32. Simulect (basiliximab) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corporation; August 2020.
33. Nulojix (belatacept) package insert. Princeton, NJ: Bristol-Myers Squibb Company; July 2021.34. Neoral (cyclosporine) package insert. East Hanover, NJ: Novartis; June 2021.
35. Gengraf (cyclosporine) package insert. North Chicago, IL: AbbVie Inc; February 2021.36. Zortress (everolimus) package insert. East Hanover, NJ: Novartis Pharmaceutical Corporation; January 2021.
37. CellCept (mycophenolate mofetil) package insert. South San Francisco, CA: Genentech USA Inc; August 2022.38. Myfortic (mycophenolate sodium) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corporation; March 2022.
39. Rapamune (sirolimus) package insert. Philadelphia, PA: Wyeth Pharmaceuticals LLC; August 2022.40. Prograf (tacrolimus) package insert. Northbrook, IL: Astellas Pharma US Inc; November 2022.
41. Envarsus XR (tacrolimus) package insert. Cary, NC: Veloxis Pharmaceuticals Inc; September 2020.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






