US Pharm. 2014;39(5):52-56.
ABSTRACT: Guidelines for the screening and management of dyslipidemias in children were published in November 2011 by the National Heart, Lung, and Blood Institute. They recommend universal screening of all children aged 9 to 11 and 17 to 21 years regardless of family history and risk factors. Despite this recommendation, pediatric practitioners do not routinely screen children without a family history or risk factor present. This article will review current guidelines for screening and treatment of dyslipidemias in children and adolescents.
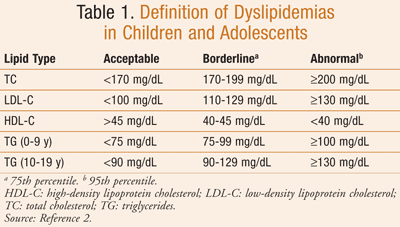
Dyslipidemias are disorders of lipoproteins that include high total cholesterol (TC), high low-density lipoprotein cholesterol (LDL-C), low high-density lipoprotein cholesterol (HDL-C), and high triglycerides (TG), all of which can contribute to atherosclerosis development and cardiovascular disease (CVD), a common cause of death in adults.1,2 The development of dyslipidemia often begins in childhood (TABLE 1),2 and as early as 9 years of age, such changes have been linked to adverse cardiac outcomes in adulthood.3 Results from several studies link development of dyslipidemias in childhood to development of CVD in adulthood.4-7
RISK FACTORS
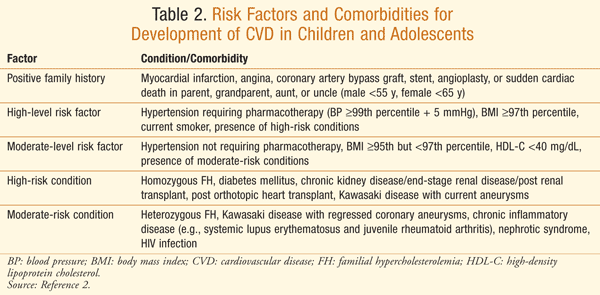
Risk factors for development of CVD in children and adolescents include genetic disorders, such as familial combined hyperlipidemia (FCH) and familial hypercholesterolemia (FH), obesity, hypertension, and diabetes. These factors place a child at high risk of subsequent development of CVD in adulthood (TABLE 2).2 Childhood obesity is a contributing factor in development of CVD risk factors.8 Moreover, prevalence of obesity in children aged 6 to 11 years has increased from 4% to over 20% in the past three decades.9 The increase in childhood obesity, along with diabetes and hypertension, heightens the importance of treating and preventing secondary causes of CVD in children and adolescents.
SCREENING
The National Heart, Lung, and Blood Institute (NHLBI), which is part of the National Institutes of Health (NIH), began the National Cholesterol Education Program (NCEP) in 1985 with a goal to “reduce illness and death from coronary heart disease in the United States by reducing the percent of Americans with high blood cholesterol.”10 In 1992, guidelines recommended screening for children and adolescents with a family history of premature CVD or elevated serum cholesterol.11 In 2008, the American Academy of Pediatrics (AAP) released guidelines for selected screening of children aged 2 to 10 years who have a family history or identified risk factor.12
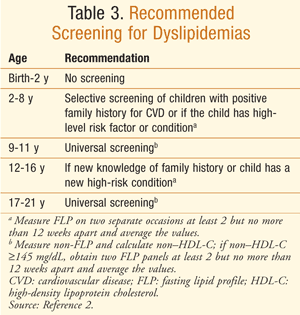
Most recently, the Expert Panel on Integrated Guidelines for Cardiovascular Risk Reduction, under a directive from the NHLBI, released guidelines for lipid screening in pediatric patients.2 Consequently, members of the expert panel decided that screening only those with a family history of dyslipidemias would fail to capture children and adolescents with atherosclerosis who are at risk for future development of CVD. Therefore, the panel recommended universal screening of all children aged 9 to 11 and 17 to 21 years (TABLE 3).2 The AAP endorsed the NHLBI guidelines, which differ from the U.S. Preventive Services Task Force (USPSTF) guidelines that concluded there was insufficient evidence to recommend universal screening.13,14
Criticisms to universal screening include lack of strong evidence and evidence from long-term clinical trials assessing efficacy of lipid-lowering therapies in children and adolescents, and potential of conflict of interest among NHLBI panel members.11,15 The USPSTF is currently revising guidelines to determine the diagnostic value of selective and universal screening of children and adolescents for treatment of dyslipidemias.16
Differences in recommendations for screening dyslipidemias in children and adolescents provide a source of confusion among practitioners. In 2012-2013, the University of Minnesota conducted an online survey over 12 weeks to assess awareness and implementation of lipid-screening guidelines among primary pediatric providers in Minnesota. Results indicated that of the 548 respondents, 34% performed no lipid screening, 50% screened selectively, and only 16% performed universal screening.17
MANAGEMENT
Lifestyle Modifications and Diet
Lifestyle modifications remain the cornerstone of dyslipidemia treatment. Goals include prompting dietary change, increasing physical activity, limiting inactivity, and achieving weight loss, if needed.2 The Cardiovascular Health Integrated Lifestyle Diet (CHILD 1) promotes healthy eating and weight. Children limit or avoid sugar-sweetened drinks, drink more water and fat-free unflavored milk, avoid foods with high trans fat and sodium, and consume high-fiber foods, fruits, vegetables, whole grains, fish, poultry, beans, nuts, and seeds. Additionally, the diet stresses control of portion sizes and overall healthy eating. If after 3 months of adherence to the CHILD-1 diet, lipid levels fail to achieve desired targets, the CHILD-2 diet specific to the abnormal lipid level is recommended. For more information about these diets, consult the NHLBI guidelines.2
Pharmacotherapy
Lifestyle modifications alone often fail to achieve desired target lipid levels, not only in adults but also in children.2 If after 6 months of lifestyle modifications and diet, desired lipid levels are not achieved, pharmacotherapy may be considered in children and adolescents who meet specific criteria stated below2:
- No CVD risk factors but has LDL-C >190 mg/dL after 6 months of lifestyle/diet changes
- CVD risk factor(s) and LDL-C ≥160 mg/dL after 6 months of lifestyle/diet changes
- Diabetes and LDL-C ≥130 mg/dL despite lifestyle/diet changes
- Average fasting TG level ≥500 mg/dL or single level ≥1,000 mg/dL.
Statins: Considered first-line therapy for treatment of dyslipidemias in children and adolescents, statins inhibit hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase, the enzyme responsible for the rate-limiting step in cholesterol synthesis. Inhibition lowers intracellular cholesterol and increases clearance of LDL-C through upregulation of LDL-C receptors. Statins lower LDL-C serum levels an average of 20% to 50%. Currently seven of the eight commercially available statins are FDA-approved as an adjunct to diet to lower LDL-C concentrations in children aged 10 to 18 years (≥8 years for pravastatin) (TABLE 4).2,8
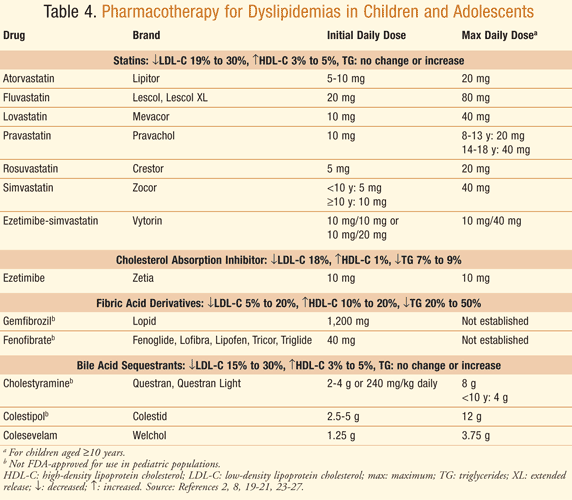
Children and adolescents with hereditary FH and LDL-C ≥190 mg/dL or who have a family history of premature CVD and two or more CVD risk factors and LDL-C ≥160 mg/dL are potential candidates for therapy.2,8 Pitavastatin is not FDA-approved for use in children or adolescents.18 Choice of statin depends upon the patient’s insurance coverage and physician preference.
Long-term safety of statins in children is unknown. Results of a recent systematic review on safety of statins in children ≤18 years showed that these drugs were well tolerated.19,20 Adverse effects were mild and included headache, gastrointestinal (GI) distress, and myalgia. Elevations in liver enzymes were infrequent, occurring in 1% to 5% of patients receiving atorvastatin and simvastatin and less frequently with lovastatin, pravastatin, and rosuvastatin.19,20 Results of a recent systematic Cochrane review in which authors identified eight trials assessing the efficacy and safety of statins for treatment of FH in children aged 7 to 17 years revealed no increased risk of liver or muscle enzyme elevations.20 Trials ranged from 6 months to 2 years. The lack of published long-term safety data requires prudent prescribing and monitoring of children requiring such therapy. Statin-induced myopathies may take weeks to months to develop.21,22 Any complaint of unusual muscle weakness or pain beginning several weeks to months after initiation of a statin should be assessed.
Cholesterol Absorption Inhibitors: This drug class inhibits cholesterol from the intestine, thereby decreasing hepatic cholesterol, which causes upregulation of LDL-C receptors and increased LDL-C clearance.2 Ezetimibe, the only cholesterol absorption inhibitor approved by the FDA, is indicated for children aged ≥10 years (TABLE 4).2,23 Addition of ezetimibe to a statin regimen lowers LDL-C levels more than statin monotherapy.23 When used in conjunction with statins, reductions in LDL-C levels of 14% to 18% may occur.2,23 Ezetimibe monotherapy when used in conjunction with diet has shown to similarly reduce LDL-C levels.24 Ezetimibe may be considered for children and adolescents who fail to meet lipid target levels on statin monotherapy, but should be used under direction of a lipid specialist. Ezetimibe is well tolerated, with adverse effects of diarrhea, arthralgia, myalgia, and nasopharyngitis reported most often.24
Fibric Acid Derivatives (Fibrates): These agents increase oxidation of fatty acids in the liver and muscle, thereby reducing the rate of hepatic lipogenesis and decreasing TG production. Although fibrates are not FDA-approved in children and adolescents (TABLE 4), use may be considered in consultation with a lipid specialist for children with TG levels >500 mg/dL.2 Fibrates should not be used in conjunction with statins unless the patient is under the care of a lipid specialist because of the increased risk of myopathy and rhabdomyolysis.2,8 In addition, gemfibrozil is a strong CYP450 inhibitor of CYP2C19, CYP2C8, and CYP2C9.25
Bile Acid Sequestrants: These medications bind bile salts in the intestine, thereby increasing the conversion of cholesterol to bile in the liver. Decreased hepatic cholesterol causes upregulation of LDL-C receptors and increased LDL-C clearance. Cholestyramine and colestipol are not FDA-approved for use in children and adolescents (TABLE 4).2,8,26 Colesevelam is indicated as monotherapy or in combination with a statin in children and adolescents with hereditary FH who have LDL-C ≥190 mg/dL or who have a family history of premature CVD and two or more CVD risk factors and LDL-C ≥160 mg/dL.2,8
Since bile salts are not absorbed systematically, major adverse effects are GI in nature and include bloating, nausea, diarrhea, and constipation. Many patients cannot tolerate these effects and stop the medication.2
Niacin: This form of vitamin B3 decreases hepatic metabolism of cholesterol and increases HDL-C concentrations. Because of the potential intolerable adverse effects, including flushing, rash, and headaches, and the limited data supporting efficacy in children, niacin should be used with caution in children and only in those under the care of a lipid specialist.2,8 Niacin may be considered for treatment of severe hypertriglyceridemia (fasting TG≥≥500 mg/dL or any single measurement of ≥1,000 mg/dL).2
Extended-release niacin products are FDA-approved in children ≥16 years. The effects of flushing, which occur more often with immediate-release formulations, may be lessened with administration of aspirin before therapy; however, routine use is linked to development of Reye syndrome.27 In addition, changing to an extended-release formulation may alleviate flushing.
Omega-3 Fatty Acids: These essential fatty acids reduce cholesterol and TG concentrations. In clinical trials with adults, 2 to 4 g daily of omega-3 fatty acids lowered TG by 30% to 40% and raised HDL-C by 6% to 17%.2 However, data supporting the use of omega-3s to treat dyslipidemias in children are sparse and have not shown beneficial effects.2,8
Researchers conducted a randomized, controlled trial in which 25 children aged 10 to 19 years with moderate-to-severe hypertriglyceridemia received omega-3s, given as Lovaza 1 g, four capsules daily or placebo for 6 months.28 Results showed a reduction in serum TG levels at 3 and 6 months of 24% with Lovaza and 16% with placebo. The reductions in TG levels between Lovaza and placebo were not statistically significant. It is worth noting that baseline TG levels ranged from 150 to 1,000 mg/dL. With the small sample size and the wide variation in baseline TG levels, more data are needed to adequately assess the benefit of omega-3 fatty acids in children with dyslipidemias.28
Other: In 2013, the FDA approved two drugs for the treatment of homozygous FH.29 Mipomersen (Kynamro) must be injected subcutaneously once weekly, and lomitapide (Juxtapid) is taken orally once daily. Both medications significantly lower LDL-C in patients with homozygous FH already taking maximum dosages of other lipid-lowering drugs. Safety and effectiveness have not been established in pediatric patients.29
PEDIATRIC VERSUS ADULT MANAGEMENT
The American College of Cardiology in conjunction with the American Heart Association released updated guidelines in November 2013 for treatment of hyperlipidemia in adults.30 These new guidelines eliminate LDL-C targets for monitoring therapy and identify four groups of adults as candidates for statin therapy30:
- Those with atherosclerotic CVD who require secondary prevention
- Those with LDL-C ≥190 mg/dL without a secondary cause
- Those aged 40 to 75 years with diabetes but no established CVD who have an LDL-C of 70 to 189 mg/dL
- Those aged 40 to 75 years who do not have diabetes or atherosclerotic CVD but who have an LDL-C of 70 to 189 mg/dL with a calculated CVD risk of ≥7.5%.
CONCLUSION
With adults, healthcare providers can calculate individual risk of CVD, which aids in guidance of lipid-lowering therapy. For children, however, initiation of pharmacotherapy is most likely for treatment of primary dyslipidemias, such as FH. The new guidelines for adults complement those for children because both state initiation of statin therapy for LDL-C ≥190 mg/dL.2,30 With the increase in secondary causes of dyslipidemias in children and adolescents, practitioners need to support lifestyle changes before initiation of lifelong lipid-lowering therapy.
REFERENCES
1. Go A, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update. Circulation. 2014;129:e28-e292.
2. National Heart, Lung, and Blood Institute (NHLBI). Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Full Report. 2011. www.nhlbi.nih.gov/guidelines/cvd_ped/index.htm. Accessed March 1, 2014.
3. Juonala M, Viikari JS, Raitakari OT. Main findings from the prospective Cardiovascular Risk in Young Finns Study. Curr Opin Lipidol. 2013;24:57-64.
4. Raitakari OT, Juonala M, Kahonen M, et al.
Cardiovascular risk factors in childhood and carotid artery intima-media
thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277-2283.
5. Morrison JA, Glueck CJ, Wang P. Childhood risk factors
predict cardiovascular disease, impaired fasting glucose plus type 2
diabetes mellitus, and high blood pressure 26 years later at a mean age
of 38 years: the Princeton Lipid Research Clinic follow-up study. Metabolism. 2012;61:531-541.
6. McGill HC Jr, McMahan CA, Zieske AW, et al. Effect of
non-lipid risk factors on atherosclerosis in youth with favorable
lipoprotein profile. Circulation. 2001;103:1546-1550.
7. Berenson GS, Srinivasan SR, Bao W, et al. Association
between multiple cardiovascular risk factors and atherosclerosis in
children and young adults. N Engl J Med. 1998;338:1650-1656.
8. Kennedy MJ, Jellerson KD, Snow MZ, Zacchetti ML.
Challenges in the pharmacologic management of obesity and secondary
dyslipidemia in children and adolescents. Pediar Drugs. 2013;15:335-342.
9. Roger VL, Go AS, Lloyd-Jones DM, et al. Executive
summary: heart disease and stroke statistics—2012 update: a report from
the American Heart Association. Circulation. 2012;125:188-197.
10. National Cholesterol Education Program. NHLBI. February 2013. https://www.nhlbi.nih.gov/about/ncep/. Accessed March 1, 2014.
11. Chauhan A, Paunikar P. Update on pediatric hyperlipidemia. Curr Opin Pediatr. 2014;26:1-7.
12. Daniels SR, Greer FR, and the Committee on Nutrition. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198-208.
13. Kavey R, Simons-Morton DG, de Jesus J, et al. Expert
panel on integrated guidelines for cardiovascular health and risk
reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213-S256.
14. U.S. Preventive Services Task Force (USPSTF).
Screening for lipid disorders in children: U.S. Preventive Services Task
Force recommendation statement. Pediatrics. 2007; 1201:e215-e219.
15. Newman TB, Pletcher MJ, Hulley SB. Overly aggressive
new guidelines for lipid screening in children: evidence of a broken
process. Pediatrics. 2012;130:349-352.
16. Draft research plan. Screening for dyslipidemia in
children and adolescents. USPSTF. February 2013.
www.uspreventiveservicestaskforce.org/uspstf14/lipiddisorders/dyslipiddraftresplan.htm.
Accessed March 1, 2014.
17. Dixon DB, Kornblum AP, Steffen LM, et al.
Implementation of lipid screening guidelines in children by primary
pediatric providers. J Pediatr. 2014;164:572-576.
18. Livalo (pitavastatin) package insert. Montgomery, AL: Kowa Pharmaceuticals America, Inc; October 2013.
19. Lamaida N, Capuano E, Pinto L, et al. The safety of statins in children. Acta Paediatr. 2013;102:857-862.
20. Vuorio A, Kuoppala J, Kovanen PT, et al. Statins for children with familial hypercholesterolemia. Cochrane Database Syst Rev. 2010;(7):CD006401.
21. Hansen KE, Hildebrand JP, Ferguson EE, Stein JH. Outcomes in 45 patients with statin-associated myopathy. Arch Intern Med. 2005;165(22):2671-2676.
22. Shannon JA, John SM, Parihar HS, et al. A clinical review of statin-associated myopathy. J Pharm Technol. 2013:29:219-230.
23. van der Graaf A, Cuffie-Jackson C, Vissers MN, et al.
Efficacy and safety of coadministration of ezetimibe and simvastatin in
adolescents with heterozygous familial hypercholesterolemia. J Amer Coll Cardiol. 2008;52:1421-1429.
24. Yeste D, Chacón P, Clemente M, et al. Ezetimibe as
monotherapy in the treatment of hypercholesterolemia in children and
adolescents. J Pediatr Endocrinol Metab. 2009;22:487-492.
25. Gemfibrozil. In: Lexi-Drugs Online. Hudson, OH:
Lexi-Comp, Inc.
http://online.lexi.com.dml.regis.edu/lco/action/doc/retrieve/docid/patch_f/6973#f_interactions.
Accessed April 15, 2014.
26. Davidson MH. A systematic review of bile acid sequestrant therapy in children with familial hypercholesterolemia. J Clin Lipidol. 2011;5:76-81.
27. Zappalla FR, Gidding SS. Lipid management in children. Endocrinol Metab Clin N Am. 2009;38:171-183.
28. de Ferranti SD, Milliren CE, Denhoff ER, et al. Using
high-dose omega-3 fatty acid supplements to lower triglyceride levels in
10- to 19-year-olds. Clin Pediatr. 2014;53:428-438.
29. Two new drugs for homozygous familial hypercholesterolemia. Med Lett Drugs Ther. 2013;55:25.
30. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013
ACC/AHA guideline on the treatment of blood cholesterol to reduce
atherosclerotic cardiovascular risk in adults: a report of the American
College of Cardiology/American Heart Association Task Force on Practice
Guidelines. Circulation. 2013 Nov 12 [Epub ahead of print].
To comment on this article, contact rdavidson@uspharmacist.com.





