US Pharm. 2013;38(5):33.
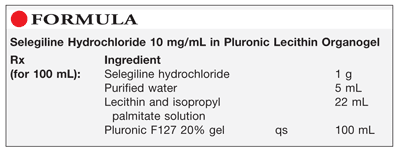
Method of Preparation: Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh or measure each ingredient. Dissolve the selegiline hydrochloride in the purified water. Incorporate the solution into about 70 mL cold Pluronic F127 20% gel and mix well. Incorporate the lecithin and isopropyl palmitate solution and mix well using a high-shear mixing method. Incorporate sufficient Pluronic F127 20% gel to volume and continue mixing using a high-shear mixing method. Package and label.
Note: The lecithin:isopropyl palmitate solution may be prepared by mixing 0.2 g sorbic acid, 50 g soy lecithin, and 50 g isopropyl palmitate. The Pluronic F127 solution may be prepared by mixing 0.2 g sorbic acid, 20 g Pluronic F127, and sufficient purified water to make 100 mL.
Use: Selegiline hydrochloride in Pluronic lecithin organogel has been used in the treatment of Parkinson’s disease and other conditions of cognitive impairment.2
Packaging: Package in a tight, light-resistant container.1
Labeling: For external use only. Use only as directed. Keep out of reach of children.
Stability: A beyond-use date of 30 days is appropriate for this preparation.1
Quality Control: Quality-control tests may include theoretical weight compared with actual weight, specific gravity, active drug assay, rheologic properties, and physical observations.2
Discussion: Selegiline hydrochloride is a relatively stereoselective monoamine oxidase type B inhibitor used in the symptomatic treatment of Parkinson’s disease, as orally administered 5-mg capsules or tablets or as a transdermal system. Many studies have reported the pharmacokinetics, effectiveness, and safety of transdermally administered selegiline over the past 15 to 20 years.3-13 The advantages of transdermal administration are the increase in blood levels of the drug and the decrease in blood levels of the metabolites, which indicate that extensive first-pass effect is avoided by topical administration. The selegiline transdermal system (Emsam; Somerset Pharmaceuticals, Inc.) is designed to deliver 6 mg, 9 mg, or 12 mg in 24 hours. The formulation presented here may be used for situations in which the commercial transdermal system may not be appropriate.
Selegiline hydrochloride (C13H17N.HCl, MW 223.74) occurs as a white, odorless, crystalline powder that is freely soluble in water and has a pKa of 7.5. It melts at 141°C to 145°C.1
Lecithin (egg lecithin, soybean lecithin, vegetable lecithin) describes a complex derived from vegetable sources. Lecithin is practically insoluble in water, polar solvents, and cold vegetable and animal oils; when mixed with water, however, it hydrates to form emulsions. It should be stored in well-closed containers protected from light.14
Isopropyl palmitate (C19H38O2, MW 298.51) is a colorless, mobile liquid that is soluble in acetone, castor oil, cottonseed oil, alcohol, and mineral oil. It is insoluble in water, glycerin, and propylene glycol. Isopropyl palmitate should be stored in well-closed containers and protected from light.15
Poloxamer 407 (Pluronic F127) is generally available in powdered form. It is odorless or may have a very mild odor. It is freely soluble in water, alcohol, and isopropyl alcohol.16
REFERENCES
1. United States Pharmacopeia 35/National Formulary 30. Rockville, MD: US Pharmacopeial Convention, Inc; 2012:344-350,1125-1126,1137,1141,1143.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of ointments/creams/gels. IJPC. 1998;2:308-309.
3. Barrett JS, Hochadel TJ, Morales RJ, et al. Pharmacokinetics and
safety of a selegiline transdermal system relative to single-dose oral
administration in the elderly. Am J Ther. 1996;3:688-698.
4. Mahmood I. Clinical pharmacokinetics and pharmacodynamics of selegiline. An update. Clin Pharmacokinet. 1997;33:91-102.
5. Rohatagi S, Barrett JS, DeWitt KE, Morales RJ. Integrated
pharmacokinetic and metabolic modeling of selegiline and metabolites
after transdermal administration. Biopharm Drug Dispos. 1997;18:567-584.
6. Sacktor N, Schifitto G, McDermott MP, et al. Transdermal
selegiline in HIV-associated cognitive impairment: pilot,
placebo-controlled study. Neurology. 2000;54:233-235.
7. Mahmood I. Selegiline transdermal system Somerset. Curr Opin Investig Drugs. 2002;3:1230-1233.
8. Bodkin JA, Amsterdam JD. Transdermal selegiline in major
depression: a double-blind, placebo-controlled, parallel-group study in
outpatients. Am J Psychiatry. 2002;159:1869-1875.
9. Patkar AA, Pae CU, Masand PS. Transdermal selegiline: the new generation of monoamine oxidase inhibitors. CNS Spectr. 2006;11:363-375.
10. Patkar AA, Pae CU, Zarzar M. Transdermal selegiline. Drugs Today (Barc). 2007;43:361-377.
11. Frampton JE, Plosker GL. Selegiline transdermal system: in the treatment of major depressive disorder. Drugs. 2007;67:257-265.
12. Goodnick PJ. Selegiline transdermal system in depression. Expert Opin Pharmacother. 2007;8:59-64.
13. Chen CC, Fang CL, Al-Suwayeh SA, et al. Transdermal delivery of selegiline from alginate-Pluronic composite thermogels. Int J Pharm. 2011;415:119-128.
14. Shah HC, Singh KK. Lecithin. In: Rowe RC, Sheskey PJ, Cook WG, Fenton ME, eds. Handbook of Pharmaceutical Excipients. 7th ed. Washington, DC: American Pharmaceutical Association; 2012:437-439.
15. Taylor AK. Isopropyl palmitate. In: Rowe RC, Sheskey PJ, Cook WG, Fenton ME, eds. Handbook of Pharmaceutical Excipients. 7th ed. Washington, DC: American Pharmaceutical Association; 2012:400-402.
16. Collett JH, Popli H. Poloxamer. In: Rowe RC, Sheskey PJ, Cook WG, Fenton ME, eds. Handbook of Pharmaceutical Excipients. 7th ed. Washington, DC: American Pharmaceutical Association; 2012:573-577.
To comment on this article, contact rdavidson@uspharmacist.com.





