US Pharm. 2006;11:HS-41-HS-42.
Linezolid
(Zyvox, Pfizer) is an oxazolidinone antibiotic indicated for the treatment of
infections caused by vancomycin-resistant enterococcus, methicillin-resistant
Staphylococcus aureus, and other aerobic gram-positive bacteria. Linezolid
inhibits protein synthesis of susceptible bacteria by binding to 23S on the
50S subunit of bacterial ribosomal RNA to prevent the formation of the
70S-initiation complex necessary for translation.1 Serious side
effects of linezolid therapy include myelosuppression and peripheral
neuropathy. However, the side effect profile of linezolid is quite favorable,
with gastrointestinal side effects most frequently reported. As linezolid
exhibits near 100% oral bioavailability, it does not require dosage conversion
from parenteral to oral regimens. Linezolid is metabolized by oxidation and is
not a substrate of the cytochrome P450 system. Clearance is primarily
nonrenal; thus, dose adjustment in patients with renal dysfunction is not
necessary, although two primary metabolites of linezolid may accumulate.1
Thus, linezolid is an attractive antibiotic choice that can easily be used on
an inpatient or outpatient basis; however, one must be aware of potential drug
interactions.
Linezolid possesses
nonspecific monoamine oxidase inhibition. Because serotonin and other
catecholamines are metabolized by monoamine oxidase, linezolid may interact
with adrenergic, serotonergic, and dopaminergic agents. It may interact with
dopamine, pseudoephedrine, selective serotonin reuptake inhibitors (SSRIs)
such as paroxetine and fluoxetine, serotonin noradrenergic reuptake inhibitors
such as venlafaxine and nefazodone, and other monoamine oxidase inhibitors
such as phenelzine and tranylcypromine. Linezolid also interacts with certain
foods, such as those containing tyramine (e.g., aged meats, cheeses, beer,
wine). Serotonergic agents promote synaptic release of serotonin or inhibit
the metabolism or reuptake of serotonin, leading to increased levels in the
central nervous system and periphery. Although toxicity from coadministration
of linezolid and serotonergic agents is not reported in phase I, II, or III
trials,2 multiple case reports describe temporally related
linezolid administration and serotonin toxicity manifesting as serotonin
syndrome.
Serotonin syndrome occurs when
there is excess central nervous system or peripheral serotonergic activity and
is characterized by altered mental status, autonomic dysfunction, and
neuromuscular abnormalities.3 It can occur with single-drug
therapy, multiple-drug therapy, or overdose. Use of recreational drugs, such
as 3,4-methylenedioxymethamphetamine (MDMA or ecstasy) and lysergic
acid diethylamide (LSD), has also been associated with serotonin syndrome.
2 Symptoms of serotonin syndrome include confusion, restlessness,
agitation, coma, seizure, lethargy, hyperthermia, tachycardia, diaphoresis,
nausea, vomiting, diarrhea, dilated pupils, hyperreflexia, myoclonus,
rigidity, trismus, and death. Laboratory tests may reveal increased white
blood cell count, increased creatinine phosphokinase, and decreased serum
bicarbonate. Early recognition and withdrawal of the offending agents is
essential.3-5 Diagnosis of serotonin syndrome is based on signs and
symptoms and medication history.3 Toxicity is treated with
supportive care, including mechanical ventilation, external cooling,
sedatives, paralytics, anticonvulsants, and antihypertensives. Cyproheptadine,
a potent antihistamine and antimuscarine antiserotonergic agent, may be used
as adjunctive therapy dosed every one to four hours (maximum daily dose of 32
mg/day.5 After withdrawal of offending medication, followed by
supportive therapy and treatment, symptoms usually resolve in 24 to 36 hours.
2
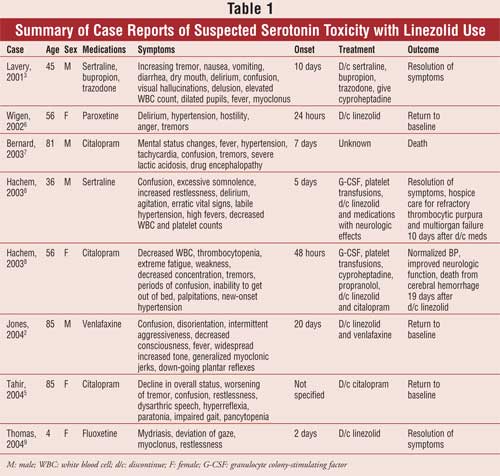
Case reports (Table 1)
describe the occurrence of serotonin syndrome in patients initiating linezolid
who were already taking serotonergic agents, including citalopram, paroxetine,
sertraline, and venlafaxine.
It is recommended in the
literature to allow two weeks between discontinuation of a serotonergic agent
and initiation of linezolid.5-8 Considering the long half-lives of
SSRIs, this recommendation is particularly prudent. For example, fluoxetine
has an elimination half-life ranging from one to six days, and an active
metabolite, norfluoxetine, may have a half-life as long as 16 days. Because of
slow elimination, interactions may occur days to weeks after discontinuation
of a serotonergic agent, depending on individual patient clearance. It is
imperative that linezolid be initiated cautiously with frequent monitoring in
patients with a recent history of serotonergic medications.
Ultimately, the recommendation
to delay start of linezolid for two weeks is not clinically realistic. A
patient with an active infection requiring linezolid therapy cannot wait two
weeks before starting an antibiotic. Dose reduction of the serotonergic agent,
although empirically reasonable, is not supported by any data. The most
reasonable strategy, also representative of clinical practice, is to use
linezolid in patients receiving serotonergic agents when the benefit outweighs
the risks. These select patients should be educated on the symptoms of
serotonin syndrome and should be closely monitored by their primary
caregivers.
Monitoring may include
complete blood counts, a chemistry panel (especially serum bicarbonate), renal
and hepatic function, mental status, and vital signs.1 Linezolid
must be discontinued and not restarted until two weeks after discontinuation
of an interacting agent, should serotonin syndrome result from a drug
interaction. Awareness of this interaction will prevent serious adverse events
and even death.
REFERENCES
1. Zyvox [package
insert]. New York, NY: Pfizer; 2005 May.
2. Jones SL, Athan E,
O'Brien D. Serotonin syndrome due to co-administration of linezolid and
venlafaxine. J Antimicrob Chemother. 2004;54:289-290.
3. Lavery S, Ravi H,
McDaniel WW, et al. Linezolid and serotonin syndrome. Psychosomatics.
2001;42:432-434.
4. Gillman PK.
Linezolid and serotonin toxicity. Clin Infect Dis. 2003;37:1274-1275.
5. Tahir N. Serotonin
syndrome as a consequence of drug-resistant infections: an interaction between
linezolid and citalopram. J Am Med Dir Assoc. 2004;5:111-113.
6. Wigen CL, Goetz MB.
Serotonin syndrome and linezolid. Clin Infect Dis. 2002;34:1651-1652.
7. Bernard L, Stern R,
Lew D, et al. Serotonin syndrome after concomitant treatment with linezolid
and citalopram. Clin Infect Dis. 2003;36:1197.
8. Hachem RY, Hicks K,
Huen A, et al. Myelosuppression and serotonin syndrome associated with
concurrent use of linezolid and selective serotonin reuptake inhibitors in
bone marrow transplant recipients. Clin Infect Dis. 2003;37:e8-11.
9. Thomas CR, Rosenberg
M, Blythe V, et al. Serotonin syndrome and linezolid. J Am Acad Child
Adolesc Psychiatry. 2004;43:790.
To comment on this article, contact
editor@uspharmacist.com.






