US Pharm. 2013;38(6)(Generic Drug Review suppl):6-10.
Prescription-drug costs account for 15% of health care expenditures in the United States. Between 2005 and 2010, the consumer price index for prescription drugs increased an average of 3.3% per year and that for total medical care costs increased by 3.8% per year, according to the Statistical Abstract of the United States, 2012.1 During the same period, annual revenue for home health care services rose by 8.6%, from $50.86 billion (2008) to $55.243 billion (2009); this is in contrast to a 6.8% increase between 2007 and 2008.2 The Centers for Medicare & Medicaid Services reported that, in 2011, the nation’s health care expenditure was $2.7 trillion, which translates to $8,680 per individual.3 However, the annual rate of increase from 2009 to 2011 remained steady at 3.9%. Health care spending as a percentage of gross domestic product held at 17.9% from 2009 to 2011. Prescription-drug sales at retail outlets increased by 0.4% in 2010 and by 2.9% in 2011, for a total of $263 billion.3 This sevenfold increase was attributed to a rise in the number and price of brand-name and specialty drugs, even with allowance for the increased use of generics (which also had price increases).
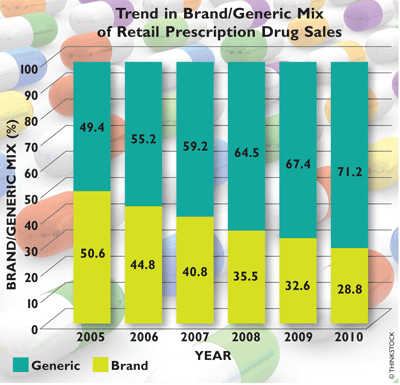
Although sales of retail prescriptions rose from $3.27 billion in 2004 to $3.7 billion in 2010 at an annual rate of about 2%, the revenue generated from retail prescriptions increased at a rate of 3.5% per annum. In 2010, 47.9% of prescriptions were dispensed in traditional chain outlets, followed by independent pharmacies (19.8%), supermarkets (13.3%), mass merchants (11.8%), and mail-order houses (7.2%), but the ranking in dollar sales across the various types of retail outlets differed. Traditional chain outlets topped the list, netting 40% of total sales revenue, followed by mail-order establishments (23.5%), independent pharmacies (16.8%), mass merchants (10%), and supermarkets (9.7%). Between 2004 and 2010, generic drugs eroded the market share of branded drugs at an average of 7.6%. In 2010, 71.2% of filled prescriptions were generics.4
Generic Drugs and Delaying Tactics
A generic drug produces the same therapeutic outcome as that of its branded counterpart. A generic drug is approved by the FDA only after the manufacturer provides data demonstrating the product to be bioequivalent to the brand-name drug that is coming off patent. Since animal study data (necessary for the Investigational New Drug Application) and data from phase I, II, and III clinical trials are not required, the cost of developing a generic version is less than that for developing a branded drug. Prompt FDA approval of Abbreviated New Drug Applications (required to market the generic version)—in comparison to the amount of time needed to obtain approval of New Drug Applications—has triggered an interest among patent holders to look for other ways to delay the entry of generic drugs.
The pay-for-delay scheme is one strategy employed by patent holders in an effort to be the sole supplier of a drug for an extended period (beyond that stipulated by rules and regulations). Such tactics for delaying the entry of generics into the market have been on the rise. That this is a trend is evident from the fact that the Federal Trade Commission received more than 100 pay-for-delay agreements between 2009 and 2011. Historically, patent holders of drugs whose patent life was about to expire made legal agreements with manufacturers who sought to enter the marketplace through litigation. These out-of-court settlements included agreements such as delayed generic entry, which allowed the patent holder to remain the sole manufacturer of the drug for a specified time. On July 16, 2012, the U.S. Court of Appeals for the Third Circuit ruled that pay-for-delay agreements were in violation of the Sherman Antitrust Act of 1890. The U.S. Supreme Court has started hearing arguments on this issue. Even though the litigation may take a couple of years to resolve, tactics designed to delay the introduction of generics may be short-lived. The growth in market share of generic drugs is four times higher than that for the pharmaceutical market as a whole.5
Short-Term Status of Generic Drug Market
Across the board, generic drugs are sold at much lower prices than those of their branded counterparts. As subsequent generics for the same brand-name drug enter the market, price wars set in. This environment helps control health care expenditures, but on the flip side gives rise to outsourcing to contract manufacturers located outside the continent.6 Contract manufacturers that deal with active pharmaceutical ingredients are known to produce goods at a substantially lower cost than in-house manufacturers.
The amount of money spent on prescription drugs is decreasing, partly because of the increased use of generics.7 The Department of Commerce has reported that the big pharmaceutical houses (innovators) have started diversifying into generics, biotechnology products, and vaccines, among other products. Generic-drug manufacturers are benefiting from the greater use of generics, but the growth in revenue from generics is short-lived. In 2012, 40 brand-name drugs generating $35 billion in sales lost patent-protection privileges, but in 2013 fewer drugs (generating $17 billion in sales) will lose their patent privileges, to the economic detriment of the generic industry.8 Between now and 2015, 41 drugs will lose their patent privileges. In 2013 and 2014, 14 and 18 drugs will come off patent, respectively; in 2015, only nine drugs will lose their patents.9 After 2015, very few breakthrough drugs will be coming off patent, so manufacturers of generic drugs will encounter a slower growth in revenue.10
Because many of the newer patented drugs are complex, their generic counterparts may not have as smooth an introduction into the market as is the case with generic versions of traditional brand-name drugs. Additionally, the FDA is beginning to take a closer look at the quality of generic drugs, which may perhaps result in further delays in the approval of generic versions of formerly patented drugs.8 Generic-drug manufacturers have been starting to reposition themselves through licensing products, globalization, specialization, and acquisitions. Faced with narrower profit margins and slow market growth, generic-drug manufacturers have taken a keen interest in exploring the development of biosimilars, which—although harder to replicate—enjoy the 12-year limit on exclusivity conferred by the Patient Protection and Affordable Care Act.10
Cost Advantage of Generics
According to the Congressional Budget Office, generic drugs save patients between $8 and $10 billion every year in retail-store sales, as well as generating savings at institutional settings such as hospitals. In 2007, the Medicare Part D program paid $60 billion for prescriptions, and 1 billion prescriptions were filled (65% filled generically). Five percent of prescriptions were filled with multiple-source brand-name drugs (i.e., brand-name drugs for which generic versions were available), and 30% were filled with single-source brand-name drugs (i.e., brand-name drugs for which no generics were available). Overall, generic drugs constituted 25% of payments made by the Medicare Part D program in 2007.11
The U.S. Census reported that, between 2004 and 2010, prescriptions for brand-name drugs were an average of 3.65 times more expensive than prescriptions for generic drugs.4 Insurers can expect to save $98 billion upon the introduction of generic versions of brand-name drugs that come off patent through 2015.12 From 2004 to 2010, the average price of generic prescriptions increased by 7.8% annually (from $28.23 to $44.14), whereas prescriptions for brand-name drugs rose by 10.48% (from $91.80 to $166.61). Taking into consideration the mix of branded and generic drugs in the prescriptions generated, however, the average increase in prescription costs from 2004 to 2010 was a modest 4%.4
Globalization of Generics
The U.S. relies on a strong generic pharmaceutical industry in order to keep the cost of prescription drugs from rising, which in turn affects national health care expenditures. According to the Department of Commerce, there has been increased globalization in both brand-name and generic drugs over the last two decades. India, China, and Israel do the most manufacturing of generics, and the fastest-growing pharmaceutical manufacturing centers are in South Korea, Brazil, the Middle East, Russia, and Southeast Asia. The Department of Commerce reports that Australia, France, Greece, Japan, and Switzerland have generic-drug utilization rates below 30%, in contrast to the U.S. rate of 86%.13 Research suggests that foreign governments’ price controls have delayed the launch of drugs in different markets, thereby adversely affecting patients’ access to new medications.
Conclusion
Millions of dollars are invested in the research, development, and marketing of drugs. Manufacturers avail themselves of patent-protection provisions to receive a reasonable return on investment. At the same time, the lower cost makes the drug more affordable. With the passage of time, generic drugs enter the market and competition affects prices, which helps reduce the cost of drug therapy for millions. Reductions in the cost of drug therapy can be further supplemented during follow-up with physicians and pharmacists. Drugs that are no longer needed require evaluation by health care professionals. Generic substitution is one of the contributors to reducing costs. For example, in the case of Medicare Part D, the dispensing of generics rather than their brand-name counterparts reduced total prescription drug costs by $33 billion in 2007. Had these substitutions not occurred, the Medicare Part D program would have incurred a 55% increased expenditure totaling $93 million.11 Therapeutic substitution, in which a higher-priced brand-name drug can be switched to a lower-priced brand-name drug in the same class, is a strategy that has helped control the rising cost of health care.
REFERENCES
1. U.S. Census Bureau. Health and Nutrition. Table 142. Consumer price indexes of medical care prices: 1980 to 2010. Statistical Abstract of the United States, 2012. www.census.gov/compendia/statab/2012/tables/12s0142.pdf. Accessed April 18, 2013.
2. U.S. Census Bureau. Health and Nutrition. Statistical Abstract of the United States, 2012.
Table 160. Annual revenue for health care industries: 2007 to 2009.
www.census.gov/compendia/statab/2012/tables/12s0160.pdf. Accessed April
18, 2013.
3. Centers for Medicare & Medicaid
Services. National health care expenditures 2011 highlights.
www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/highlights.pdf.
Accessed April 18, 2013.
4. U.S. Census Bureau. Health and Nutrition. Statistical Abstract of the United States, 2012.
Table 159. Retail prescription drug sales: 1995 to 2010.
www.census.gov/compendia/statab/2012/tables/12s0159.pdf. Accessed April
14, 2013.
5. Stone K. Are pay-for-delay
settlements history? About.com.
http://pharma.about.com/od/Government_IP/a/Are-Pay-For-Delay-Settlements-History.htm.
Accessed April 18, 2013.
6. Harachand S. A twist in generics sales: are Indian CMO factories the key to global forays? Contract Pharma. www.contractpharma.com/issues/2007-09/view_india-report/a-twist-in-generics-sales. Accessed March 3, 2013.
7. Thomas K. U.S. drug costs dropped in 2012, but rises loom. New York Times.
March 18, 2013.
www.nytimes.com/2013/03/19/business/use-of-generics-produces-an-unusual-drop-in-drug-spending.html?pagewanted=all&_r=0.
Accessed April 18, 2013.
8. Thomas K. Generic drug makers see a drought ahead. New York Times.
December 3, 2012.
www.nytimes.com/2012/12/04/business/generic-drug-makers-facing-squeeze-on-revenue.html?pagewanted=all&_r=0.
Accessed March 3, 2013.
9. Corporate Pharmacy Services. Upcoming
generic drugs. www.corporatepharmacy.com/page/upcoming_generic_drugs.
Accessed April 18, 2013.
10. Alazraki M. The 10 biggest-selling
drugs that are about to lose their patent.
www.dailyfinance.com/2011/02/27/top-selling-drugs-are-about-to-lose-patent-protection-ready.
Accessed April 18, 2013.
11. Congressional Budget Office. Effects
of using generic drugs on Medicare’s prescription drug spending.
www.cbo.gov/publication/21800. Published September 2010. Accessed April
19, 2013.
12. Pierson R. Generics seen slashing global drug sales
growth.
www.reuters.com/article/2011/05/18/us-pharmaceuticals-forecast-idUSTRE74H0M420110518.
Reuters. New York, NY: May 18, 2011. Accessed April 18, 2013.
13. U.S. Department of Commerce, International Trade Administration. Pharmaceutical
Price Controls in OECD Countries: Implications for U.S. Consumers,
Pricing, Research and Development, and Innovation. Washington DC: International Trade Administration; 2004:23.
To comment on this article, contact rdavidson@uspharmacist.com.






